Learning new skills that your organization needs to thrive and succeed today also gives you the potential to progress within your career and boost your employability.
In this article we outline three key skills for validation professionals working in the life sciences industry in 2022:
1. Critical thinking
2. Technical know-how
3. Time management

1. Critical thinking
Critical thinking skills are sometimes described as ‘higher order’ skills—namely, skills requiring ways of thinking that are deeper and more complex than the kind of thinking that we use every day.
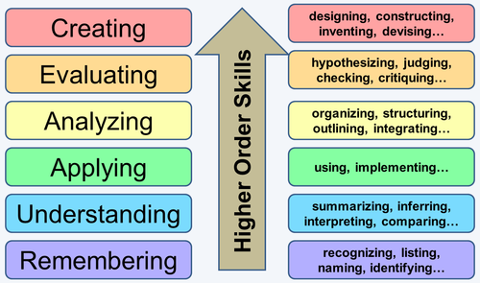
Critical thinking skills include the ability to analyze and evaluate the information that you encounter during your work, and then create inferences or conclusions based upon your analysis and evaluation.
Encouraged by the FDA’s emerging ‘commonsense’ regulatory outlook, the GAMP® 5 framework it’s underpinned by, and the ever-increasing volume of computer-systems involved in manufacturing, the role of critical thinking and risk-based prioritization will grow in 2022.
The FDA’s novel approach to Computer Systems Validation (CSV)—Computer Software Assurance (CSA) —represents a step-change in computer system validation, placing a risk-based approach and critical thinking at the center of the CSV process, as opposed to a traditional almost ‘one size fits all’ approach.
CSA offers many benefits to the life sciences industry. It will reduce validation cost and time by focusing on the software’s impact on patient safety, impact on product quality, and impact on quality system integrity (Direct or Indirect system).
The FDA’s Center for Devices and Radiological Health (CDRH) intends to publish its draft guidance on Computer Software Assurance for Production and Quality System Software in 2022. (1) The FDA’s guidance on CSA is eagerly awaited and will result in a re-boot of quality best-practice. The guidance aims to reduce the burden of CSV, improve quality, remove non-value-added activities, and focus testing on high-risk areas only.

2. Technical know-how
As software overtakes paper as the de facto standard for performing validation, relevant technical know-how will become a key skill for validation and quality assurance professionals around the world. Kneat’s cloud-based validation software platform, Kneat Gx, is used by Engineers to help them perform their work effectively, releasing them from paper; by Managers to create, maintain and manage best practice processes; by Quality Directors to oversee quality and satisfy compliance; and by CIOs to help deliver digital transformation. Today, our software is trusted by eight of the world’s top ten pharmaceutical companies and has more than 17,000 active users globally.
The recent rapid adoption of Kneat Gx by life sciences—and other highly regulated industries—is the ethos behind why Kneat innovated and created Kneat Academy. Founded in 2020, Kneat Academy is the accredited peak training body for Kneat Gx today.
Dedicated to ‘training the future of validation’ through a robust syllabus of certified Kneat Gx courses, Kneat Academy provides training for all competency levels, from fundamental to advanced, for students to seasoned professionals.
Located across Europe and North America, Kneat Academy’s proven and highly experienced Instructor Team are certified to the highest Kneat Academy certification level and have educated thousands of trainees in over 20 countries in multiple languages. The team is dedicated to delivering the highest quality training, building Kneat Academy’s reputation as the leading provider of validation software training in the world.
Kneat Academy’s training programs enable you to stay ahead of industry change and future-proof your validation or quality assurance career.
3. Time management
Validation projects often directly impact other work and contribute to speed to market within an organization. The importance of being able to manage your time to commit to deadlines and successfully complete validation projects quickly, can be a critical factor in the overall performance and competitiveness of a business.
Kneat gets you instant visibility, at a macro and micro level, into every aspect of the validation process across all your facilities in real-time, in one place, from anywhere in the world. You can instantly see if a system is validated, due for review, or in review, and check the status of all documents and exceptions per system, project, site, and organization—in a single click. Enabling you to monitor, prioritize, and manage your validation workflow effectively and efficiently.
 Prepare for the future of validation
Prepare for the future of validation
Today, the where, when, and how of validation is changing. The advent of new regulations in 2022, like the CSA, have amplified the need for critical thinking skills, whilst the wide adoption of new validation software by highly regulated industries, such as the life sciences sector, requires dedicated software training, which in turn, can help to underpin effective time management skills and remote teamwork.
References
- CDHR Proposed Guidances for Fiscal Year 2022, [https://www.fda.gov/medical-devices/guidance-documents-medical-devices-and-radiation-emitting-products/cdrh-proposed-guidances-fiscal-year-2022-fy2022].







