Enhance compliance and efficiency
Kneat digitalizes the entire commissioning & qualification (C&Q) validation lifecycle. Delivering significant productivity improvements and assuring compliance with regulatory standards.
Implement risk-based approaches
Kneat facilitates a risk-based, lean, lifecycle approach to C&Q that is aligned with ASTM E2500 best practice methods.
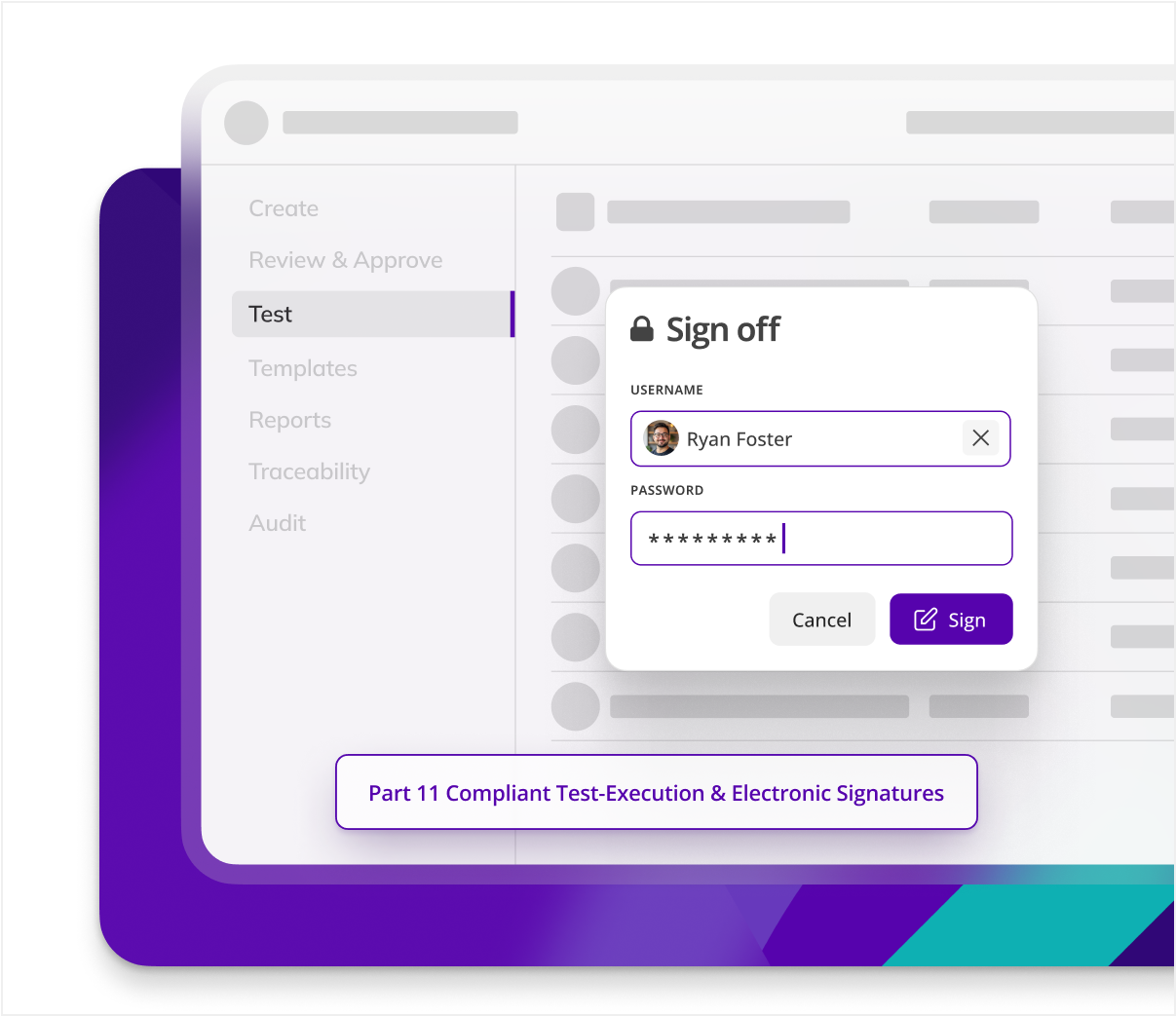
Eliminate paper records
By digitalizing C&Q processes, Kneat eliminates 100% of paper records, reducing physical storage needs and associated costs, while also minimizing protocol-based GDP errors.
Gain real-time process visibility
Kneat provides instant macro and micro visibility into all aspects of the C&Q process in real time, enabling better decision-making and proactive issue resolution.
Trusted By
Customer Success
Case studies

Before, we had Veeva....We had a lot of documentation, but it did not have the level of organization and accessibility that we have with Kneat. We also couldn’t execute C&Q digitally.
- Global Director, Commissioning & Qualification
Book a demoResults
MORE EFFICIENT FOR c&Q THAN COMPETITOR
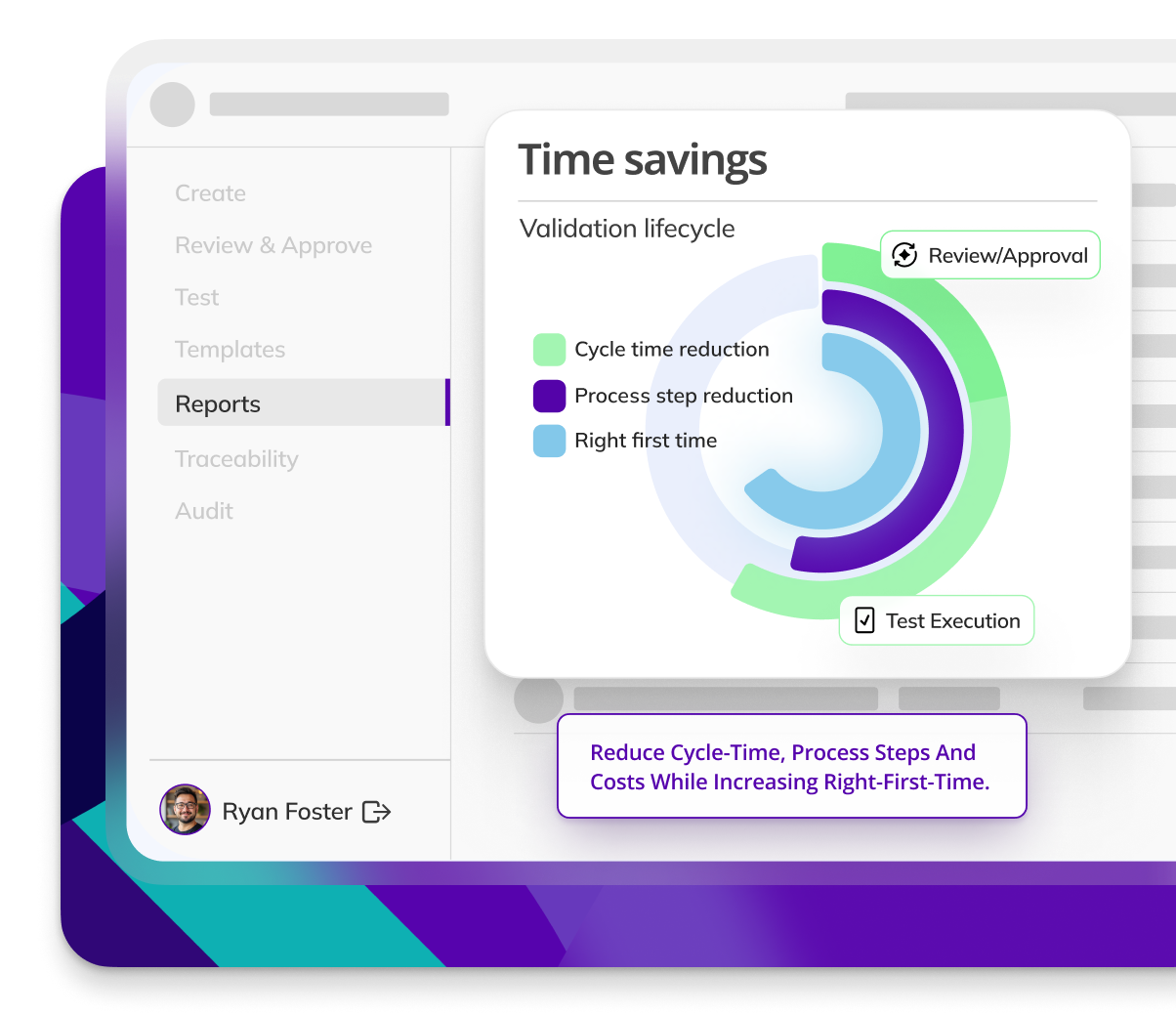
Kneat is proven to be 40% more efficient for commissioning & qualification than a leading competitor product. As found in a customer comparative pilot study.
urs approval cycle time reduction
Kneat is proven to reduce user requirements specification approval cycle time by 88%. As found in a customer authored case study.
Reduction in Review-Approval Time
Kneat is proven to reduce review-approval cycle time by 40%. As found in a customer authored case study.
Revolutionize your validation
Digitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Book a demo