Validate, anywhere
Kneat’s 100% digital platform, accessible via tablet, enables seamless validation of temperature-controlled areas across your facility and supply chain. Assuring compliance anytime, anywhere.
Safeguard the cold chain
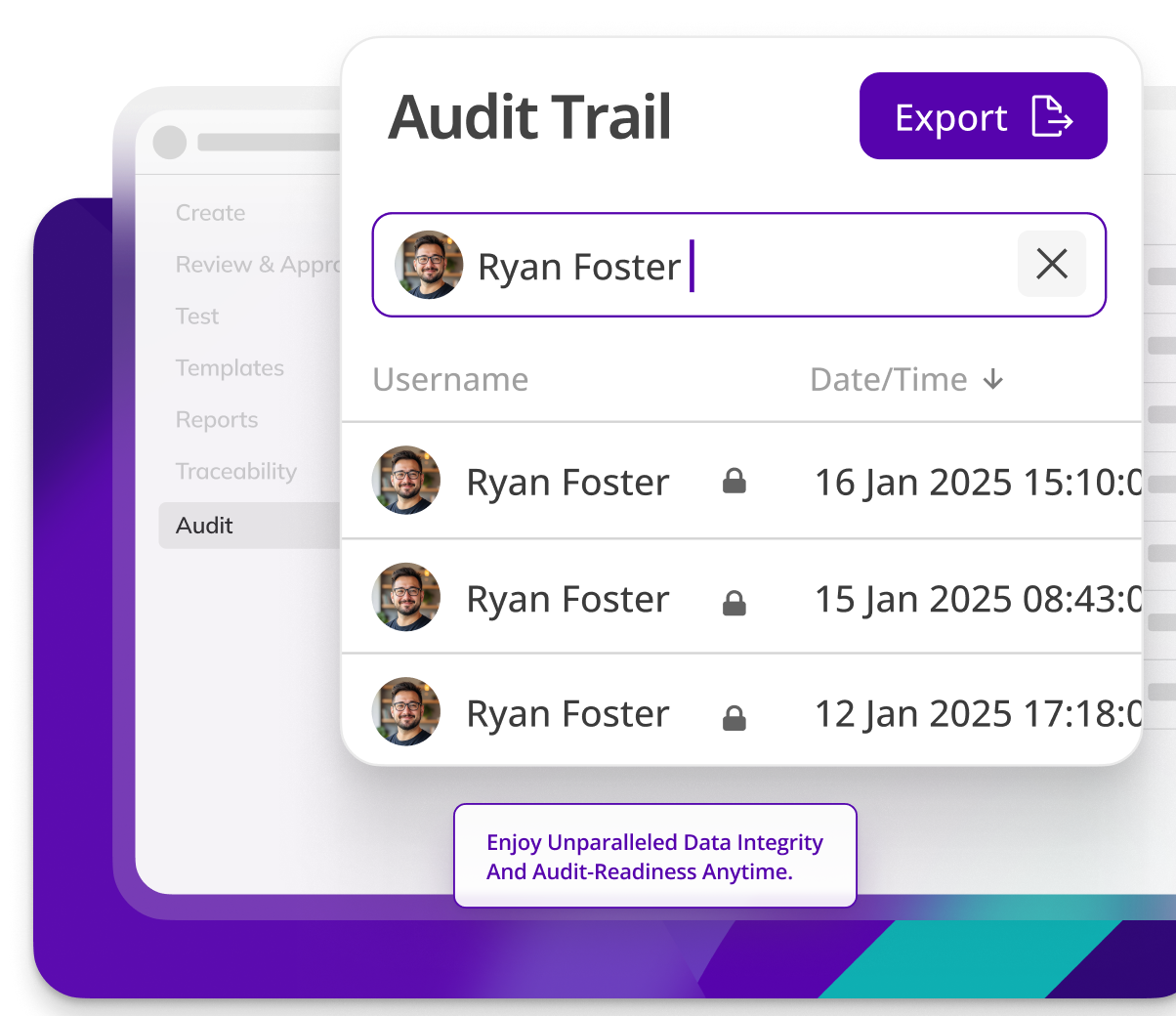
Kneat’s data integrity controls protect the cold chain, assuring temperature records are accurate and tamper-proof. Minimize the risk of temperature and protecting against product loss.
Comply across borders
Kneat’s flexible platform empowers users to quickly adapt cold chain validation processes to meet evolving global regulations. Easily update workflows and assure compliance across dynamic supply chains.
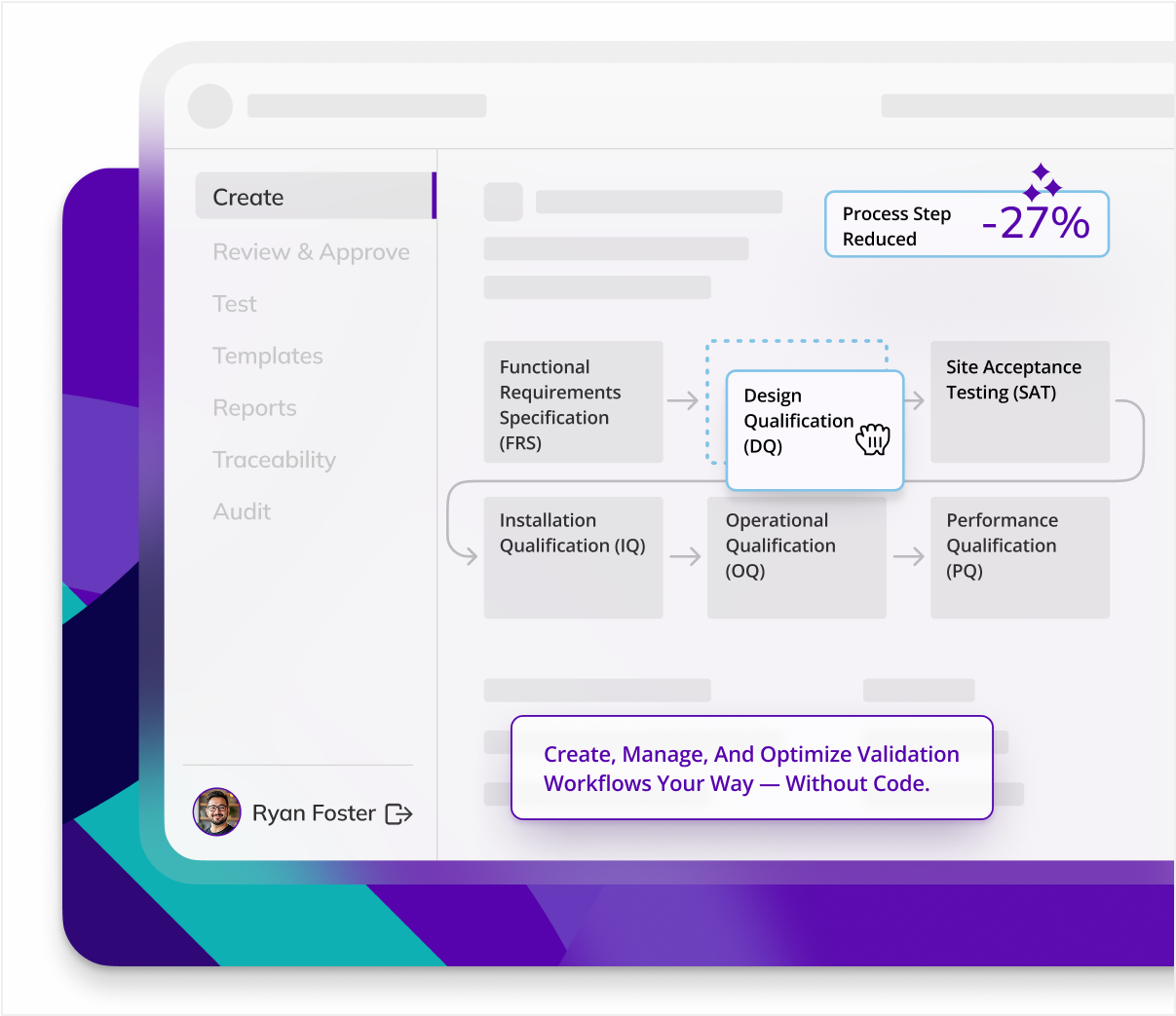
Drive end-to-end efficiency
Documenting and validating the entire cold chain process, including storage, handling, and transport, is time-consuming and prone to errors when done manually.
Trusted By
Customer Success
Case studies

Did we get value by implementing Kneat? ... It’s a no-brainer. We’re seeing gains in schedule and work hours, positive feedback from users, and improvements to core metrics.
- Global Engineering, C&Q, Top 10 Biopharmaceutical Company
Book a demoResults
CYCLE TIME REDUCTION
Kneat is proven to reduce cycle time by an average of 50% or more in multiple customer authored case studies.
CSAT Score
97% of our customers rate our customer support as either ‘Very Good’ or ‘Excellent.’
PLAN TO RENEW
Customer feedback via industry analyst SoftwareReviews.
Revolutionize your validation
Digitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Book a demo