Efficient change control
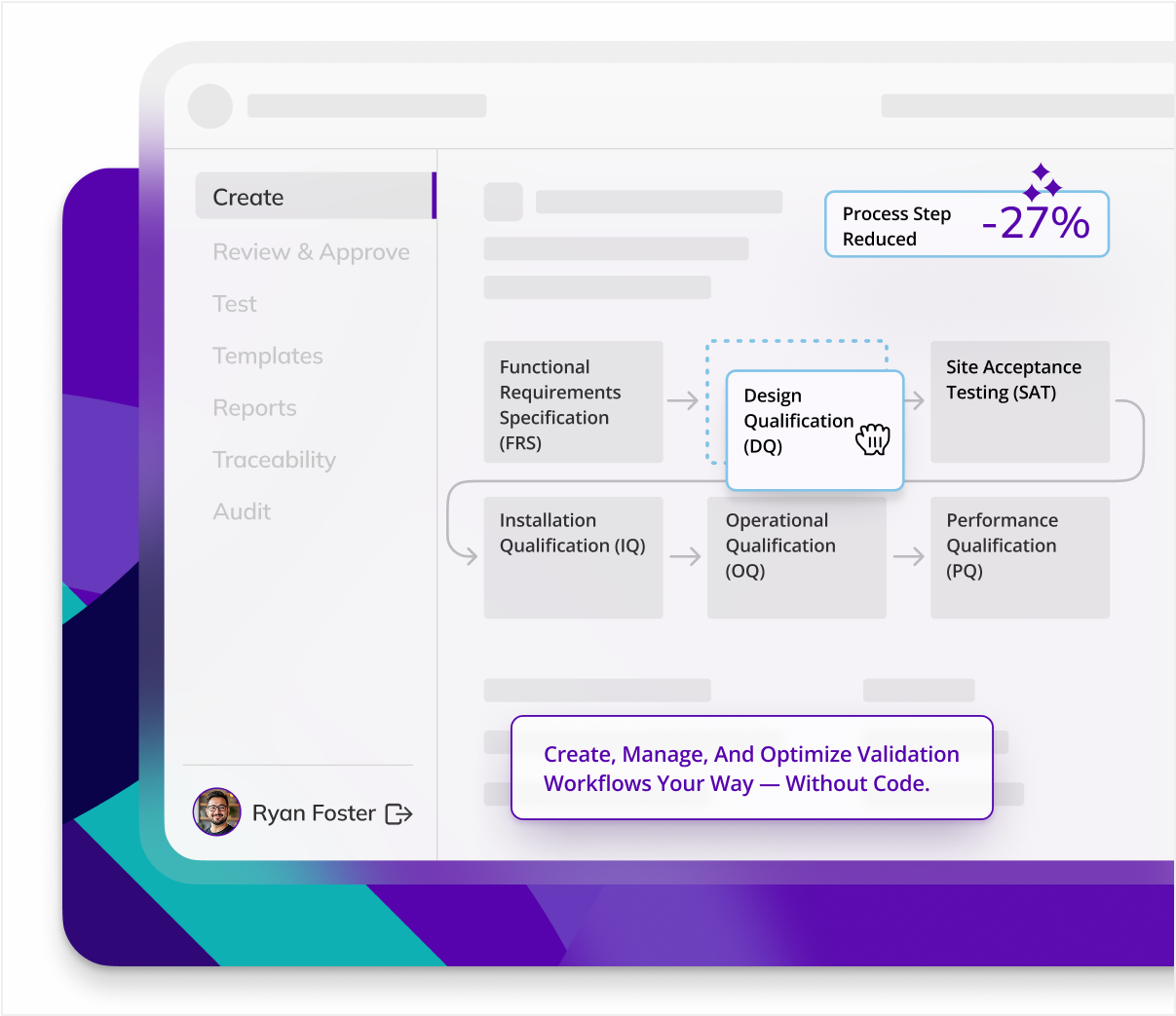
Frequent software updates and evolving business needs force validation teams into continuous revalidation cycles, straining resources and increasing risk. Kneat streamlines change control enabling you to reduce change-assessment turnaround and change-implementation time.
Ensure data integrity
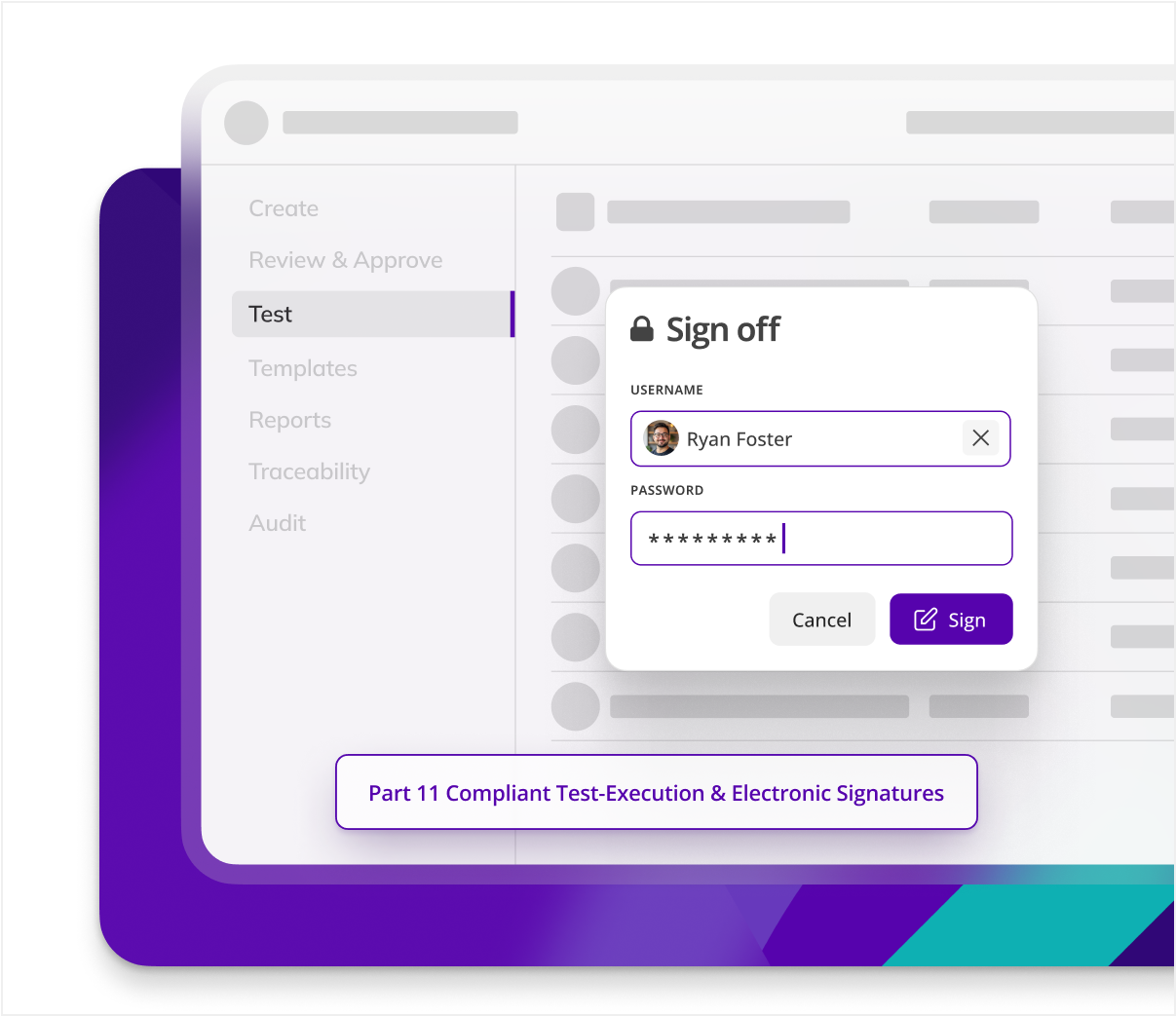
Data integrity is often challenging, yet critical to maintain. Kneat enforces compliance through secure and traceable data integrity checks, safeguarding regulatory adherence.
Reach industry 4.0 faster
Kneat accelerates computer software assurance by reducing review-approval cycle times and supporting flexible, risk-based testing. Ensuring validation professionals keep pace with Industry 4.0’s expanding system landscape.
Remote freedom
Kneat enables 100% remote computer systems validation, reducing deployment time and costs for global systems.
Trusted By
Customer Success
Case studies

It’s what I really wanted, the ability to still author and execute in the traditional manner, but not having the ‘on paper’ anymore.
Manager IT Compliance, Fujirebio Diagnostics, Inc
Book a demoResults
CYCLE TIME REDUCTION
Kneat is proven to reduce cycle time by an average of 50% or more in multiple customer authored case studies.
CSAT Score
97% of our customers rate our customer support as either ‘Very Good’ or ‘Excellent.’
PLAN TO RENEW
Customer feedback via industry analyst SoftwareReviews.
Revolutionize your validation
Digitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Book a demo