Track critical parameters
Kneat enables organizations to define, monitor, and document critical process parameters, simplifying compliance and ensuring consistent process outcomes.
Manage changes easily & securely
Kneat allows for controlled changes to validated processes, ensuring all modifications are documented and approved without disrupting operations or compliance.
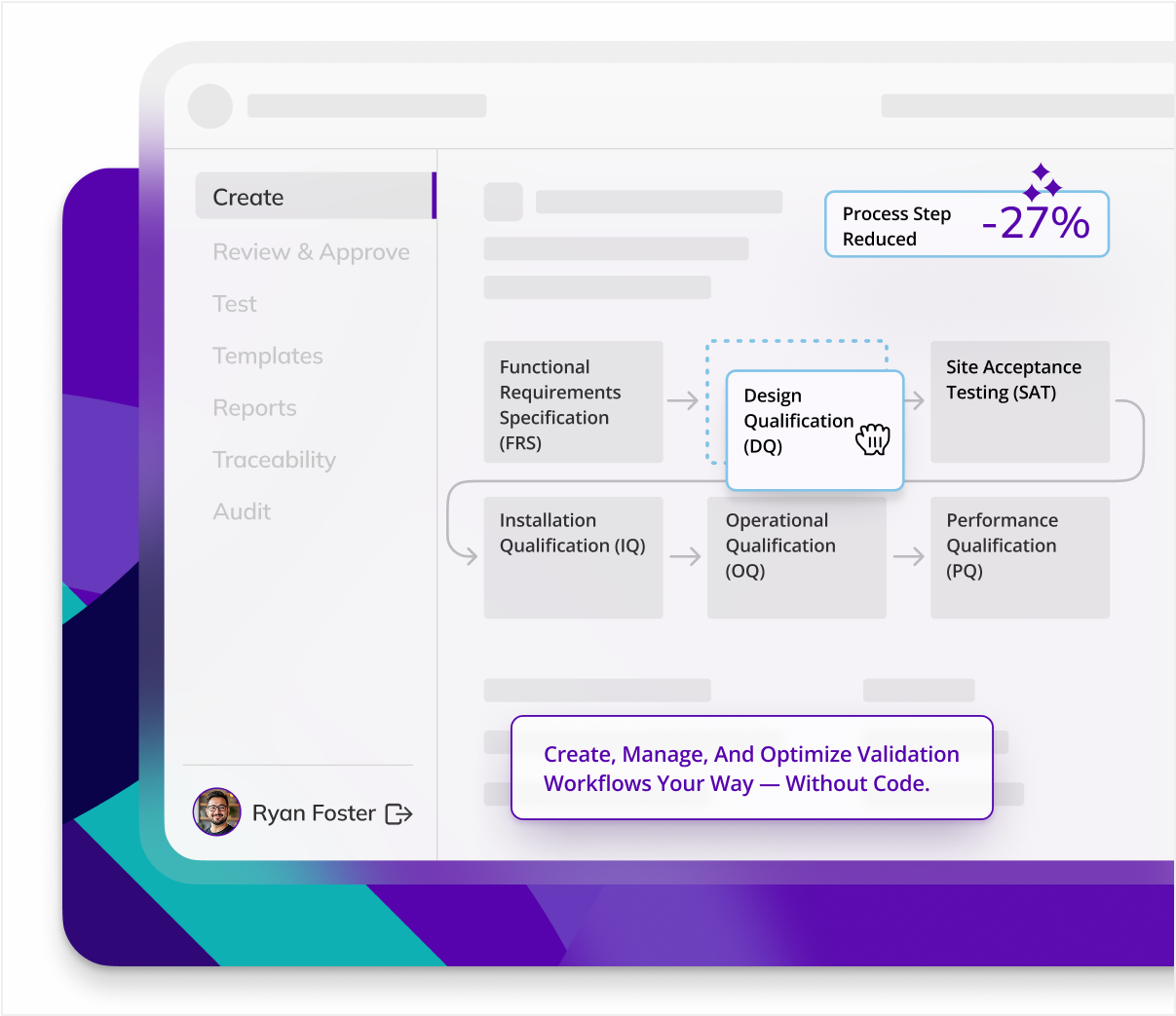
Centralized validation
Kneat centralizes all validation processes and data in a single solution, sreamlining process validation by enabling seamless linking of data and documents.
Higher right first time success
Kneat enables a right-first-time approach with robust data integrity controls that reduce error and prevent delay.
Trusted By
Customer Success
Case studies

The flexibility of Kneat is huge ... if we want to onboard a new client, it’s a 15-minute meeting between us, and we set it up how we want. We update our process mapping document, and we move on.
- Laura Berberian, Manager, Quality Assurance Validation, ElevateBio
Book a demoResults
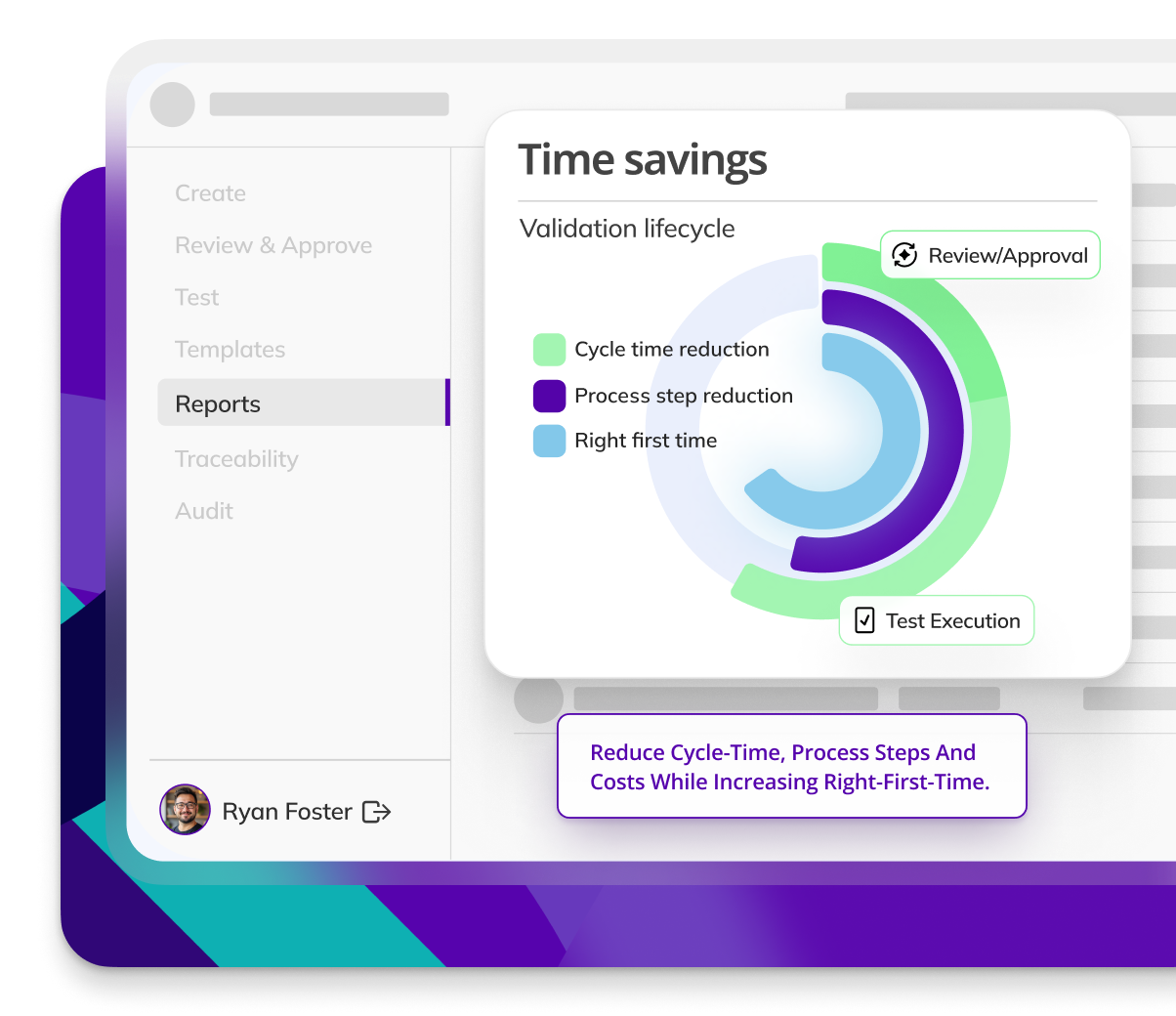
CYCLE TIME REDUCTION
Kneat is proven to reduce cycle time by an average of 50% or more in multiple customer authored case studies.
CSAT Score
97% of our customers rate our customer support as either ‘Very Good’ or ‘Excellent.’
PLAN TO RENEW
Customer feedback via industry analyst SoftwareReviews.
Revolutionize your validation
Digitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Book a demo