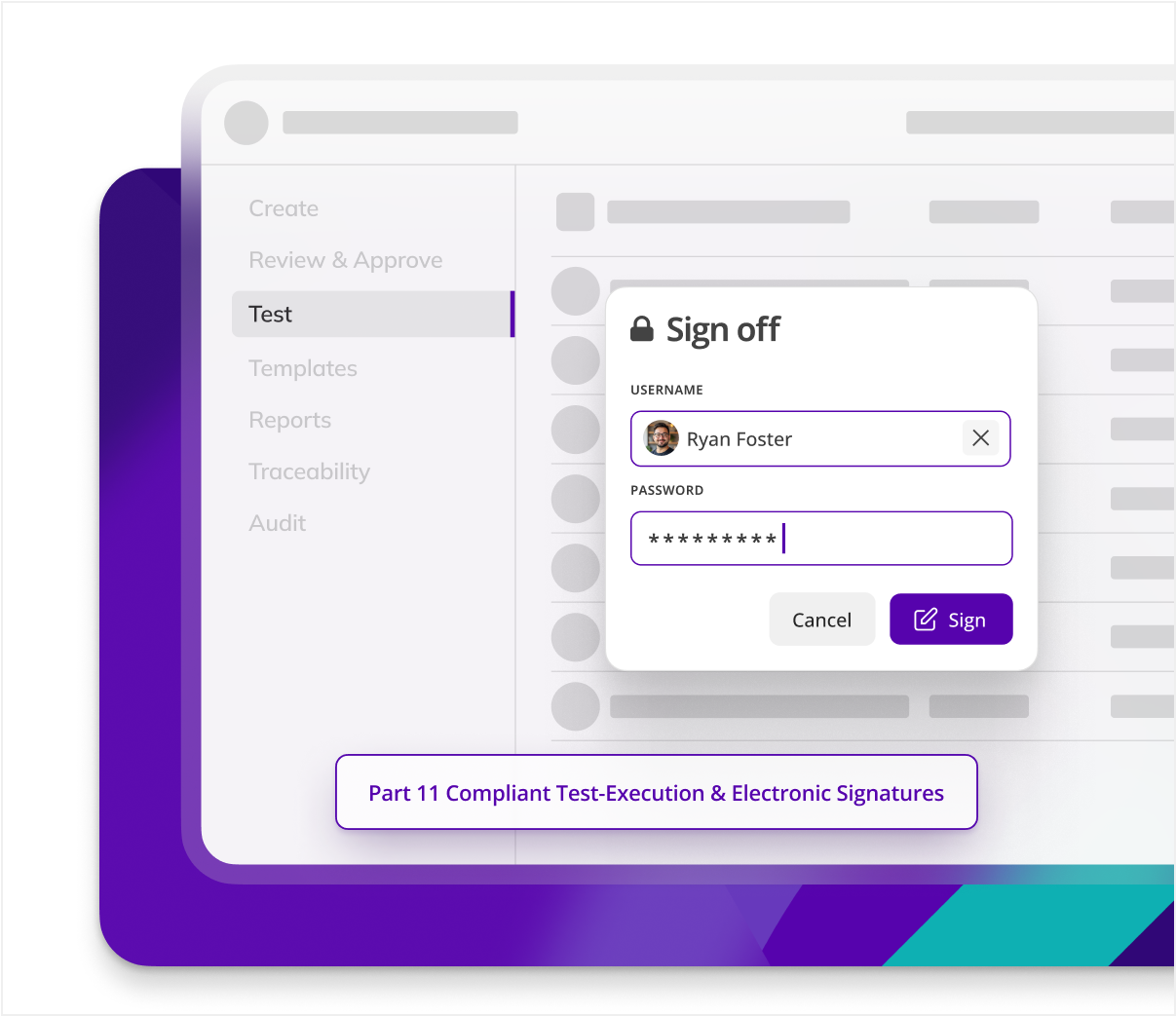
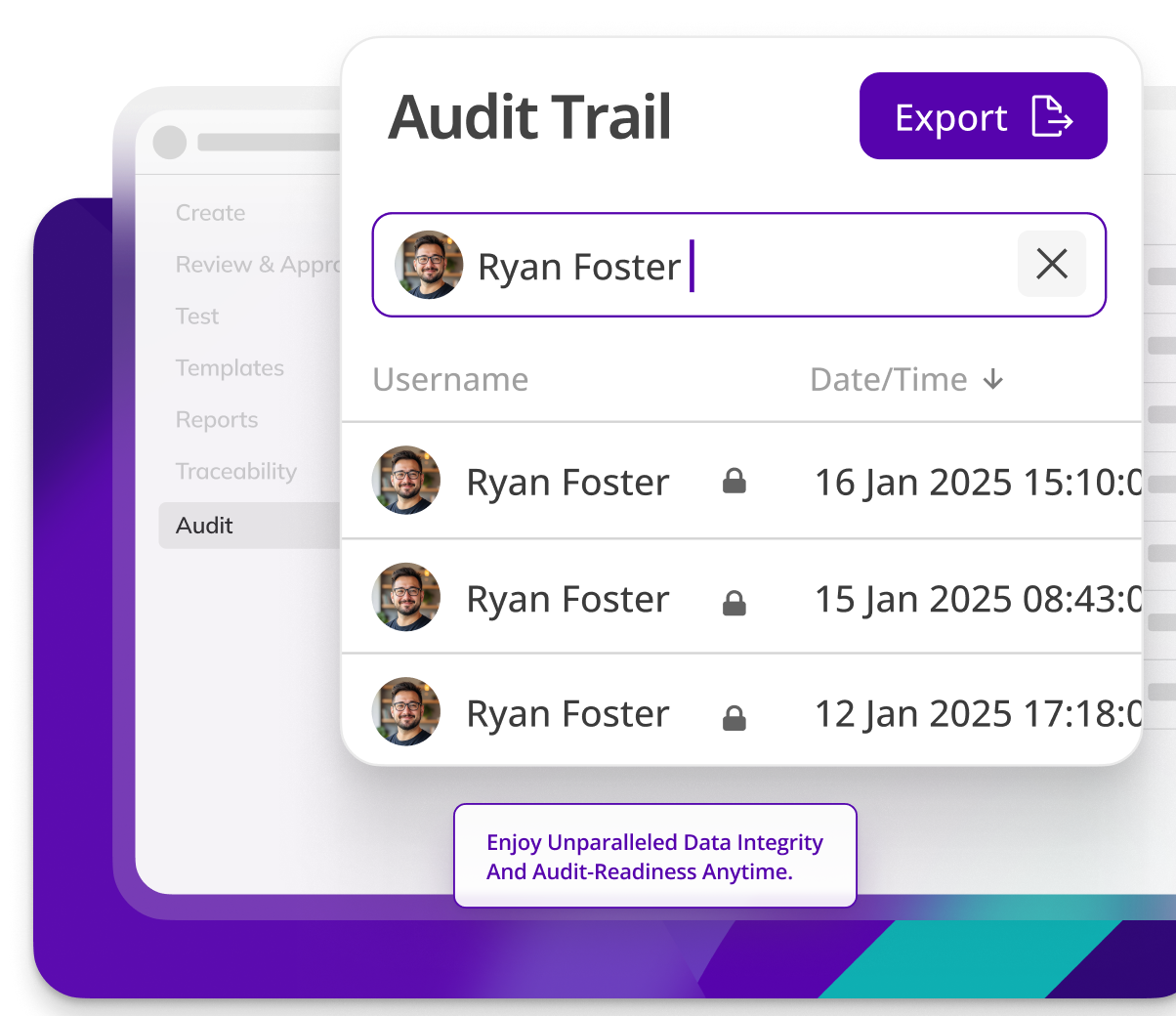
Faster non-conformance resolution
Audits demand rapid non-conformance traceability and resolution. Kneat centralizes data to enable instant tracking, analysis, and resolution to make compliance faster and more efficient.
Effortless audit preparation
Preparing for audits is time-intensive. Kneat eliminates manual document recall, ensuring you’re always audit ready. With Kneat’s powerful “Collections” feature, you gain full control over when and what auditors see.
Stay ahead of regulations
Evolving regulations create uncertainty. Kneat assures up-to-date compliance by automating regulatory checks and flagging changes to keep you aligned with industry standards.
Proactive risk tracking
Unidentified risks can delay validation and compliance. Kneat’s digital validation software enables real-time risk tracking, enforcing consistent processes to mitigate issues early and assure compliance.
Trusted By
Customer Success
Case studies

It’s what I really wanted, the ability to still author and execute in the traditional manner, but not having the ‘on paper’ anymore.
Manager IT Compliance, Fujirebio Diagnostics, Inc
Book a demoResults
CYCLE TIME REDUCTION
Kneat is proven to reduce cycle time by an average of 50% or more in multiple customer authored case studies.
CSAT Score
97% of our customers rate our customer support as either ‘Very Good’ or ‘Excellent.’
PLAN TO RENEW
Customer feedback via industry analyst SoftwareReviews.
Revolutionize your validation
Digitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Book a demo