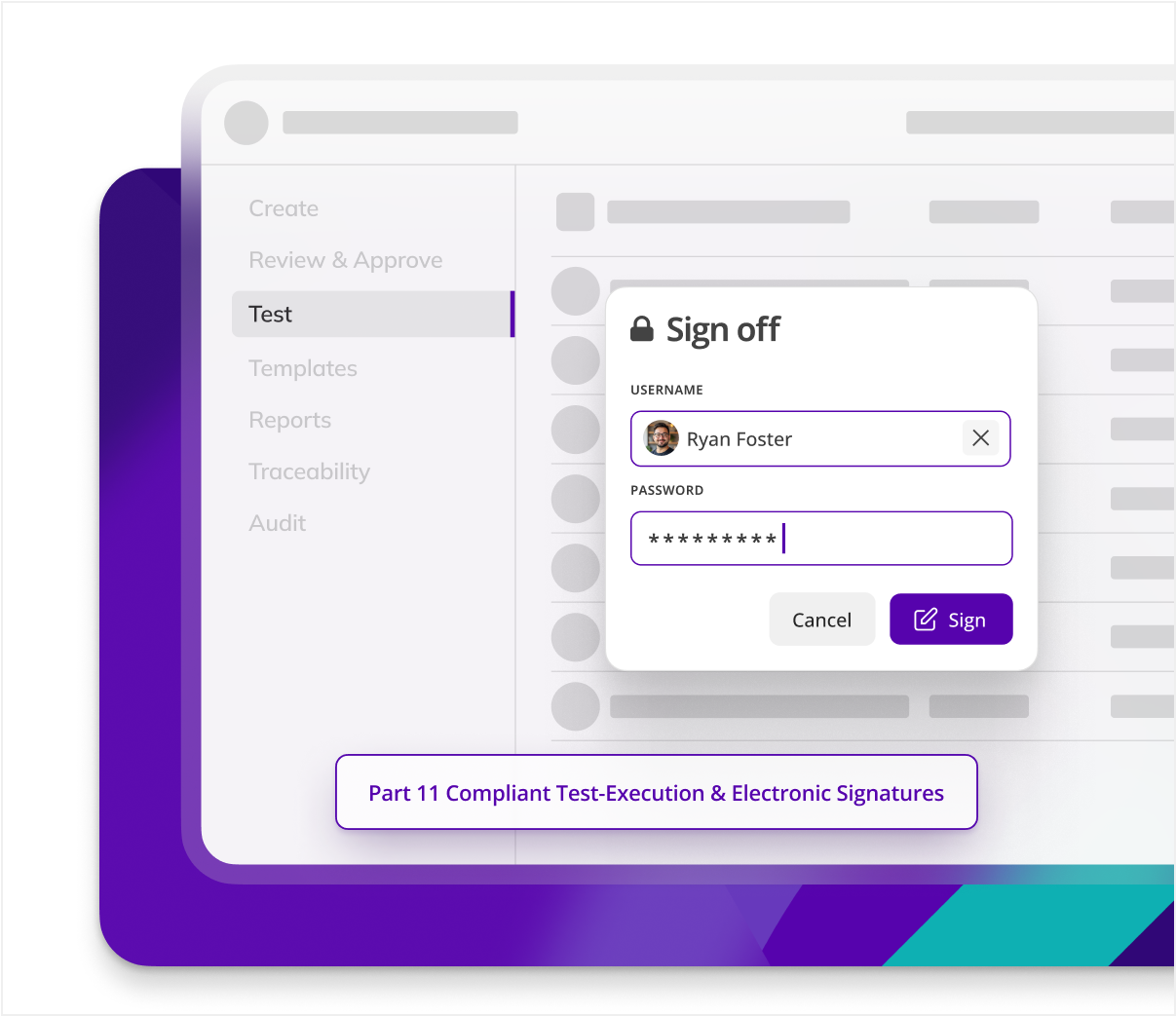
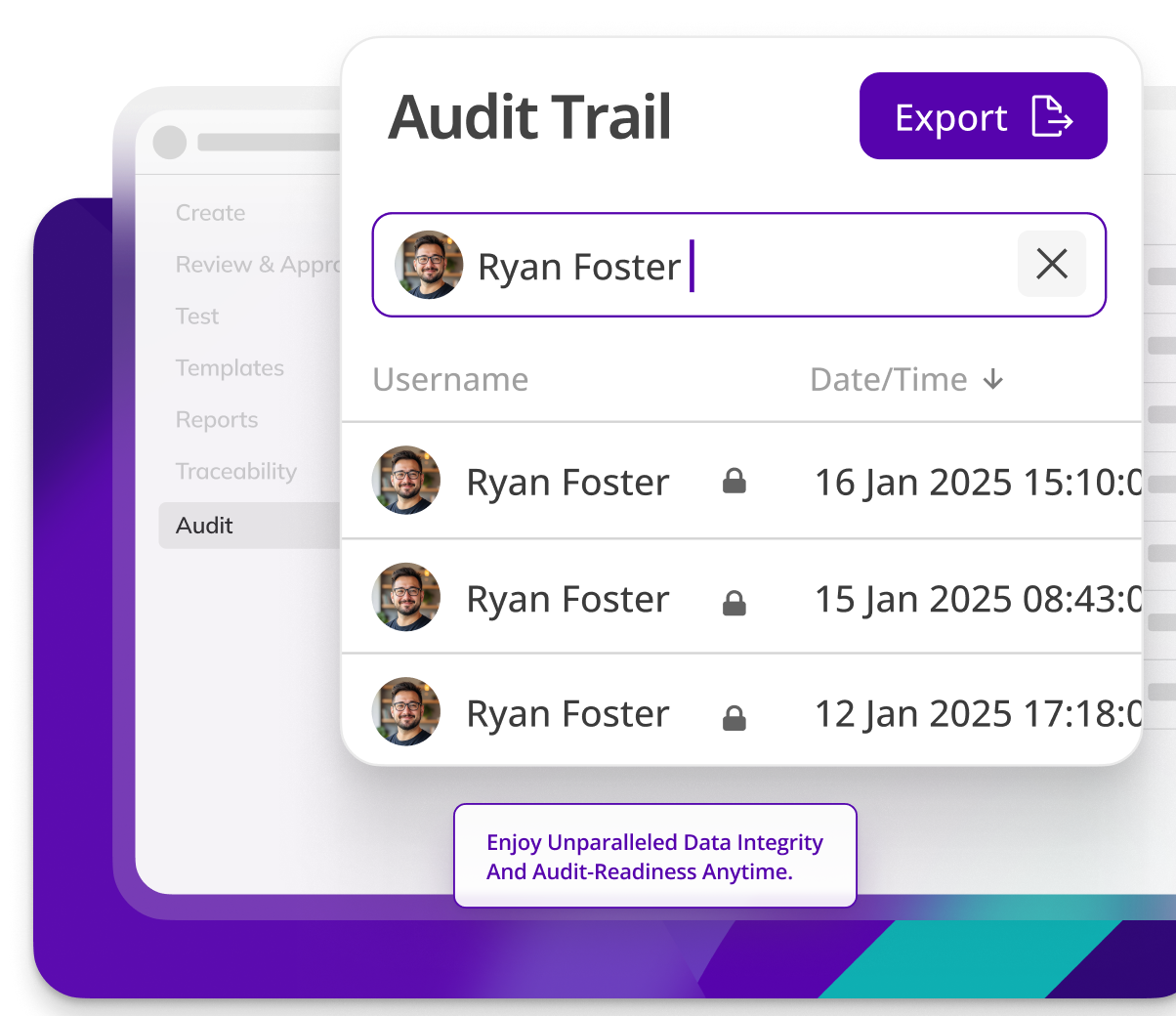
Enhance document integrity
Kneat protects the integrity of your documents automatically. Enforcing document check-out for secure edits, Part 11 compliant electronic signatures, user access controls, automated version control, and a comprehensive audit trail.
Integrate doc-management & validation
Solve document management and validation in one solution. Easily associate any document to your validation protocol as test evidence. Store, search, retrieve, trace, and link GxP and non-GxP documents and validation protocols in one system.
Simplify version control
Kneat automates version control, ensuring teams always work with the latest approved documents throughout the validation lifecycle, reducing errors and delays.
Streamline approvals
Kneat enables online collaboration with annotation tools and redlining. Simplify reviews and accelerating approvals, while ensuring compliance.
Trusted By
Customer Success
Case studies

It’s what I really wanted, the ability to still author and execute in the traditional manner, but not having the ‘on paper’ anymore.
Manager IT Compliance, Fujirebio Diagnostics, Inc
Book a demoResults
CYCLE TIME REDUCTION
Kneat is proven to reduce cycle time by an average of 50% or more in multiple customer authored case studies.
CSAT Score
97% of our customers rate our customer support as either ‘Very Good’ or ‘Excellent.’
PLAN TO RENEW
Customer feedback via industry analyst SoftwareReviews.
Revolutionize your validation
Digitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Book a demo