Reduce contamination risks
Cross-contamination risks are high in multi-product facilities. Kneat digitalizes protocols to enforce consistent cleaning practices and significantly reduce contamination risks.
Ensure complete traceability
Manual cleaning validation records can be inconsistent and error-prone. Kneat digitalizes documentation to enhance accuracy, ensure traceability, and streamline compliance with regulatory standards.
Scalable cleaning processes
Scaling cleaning protocols often disrupts operations. Kneat simplifies scalability by automating process validation and tracking, allowing for seamless growth.
Eliminate manual errors
Manual tracking frequently leads to errors. Kneat eliminates these with automated, validated workflows that assure consistent cleaning compliance across operations.
Trusted By
Customer Success
Case studies

We were able to demonstrate over 50% cycle time reduction…and process simplification from 15 steps to 8 … because of Kneat we minimized the number of systems we used from 5 to 2.
- Global Executive Director, MSD
Book a demoResults
CYCLE TIME REDUCTION
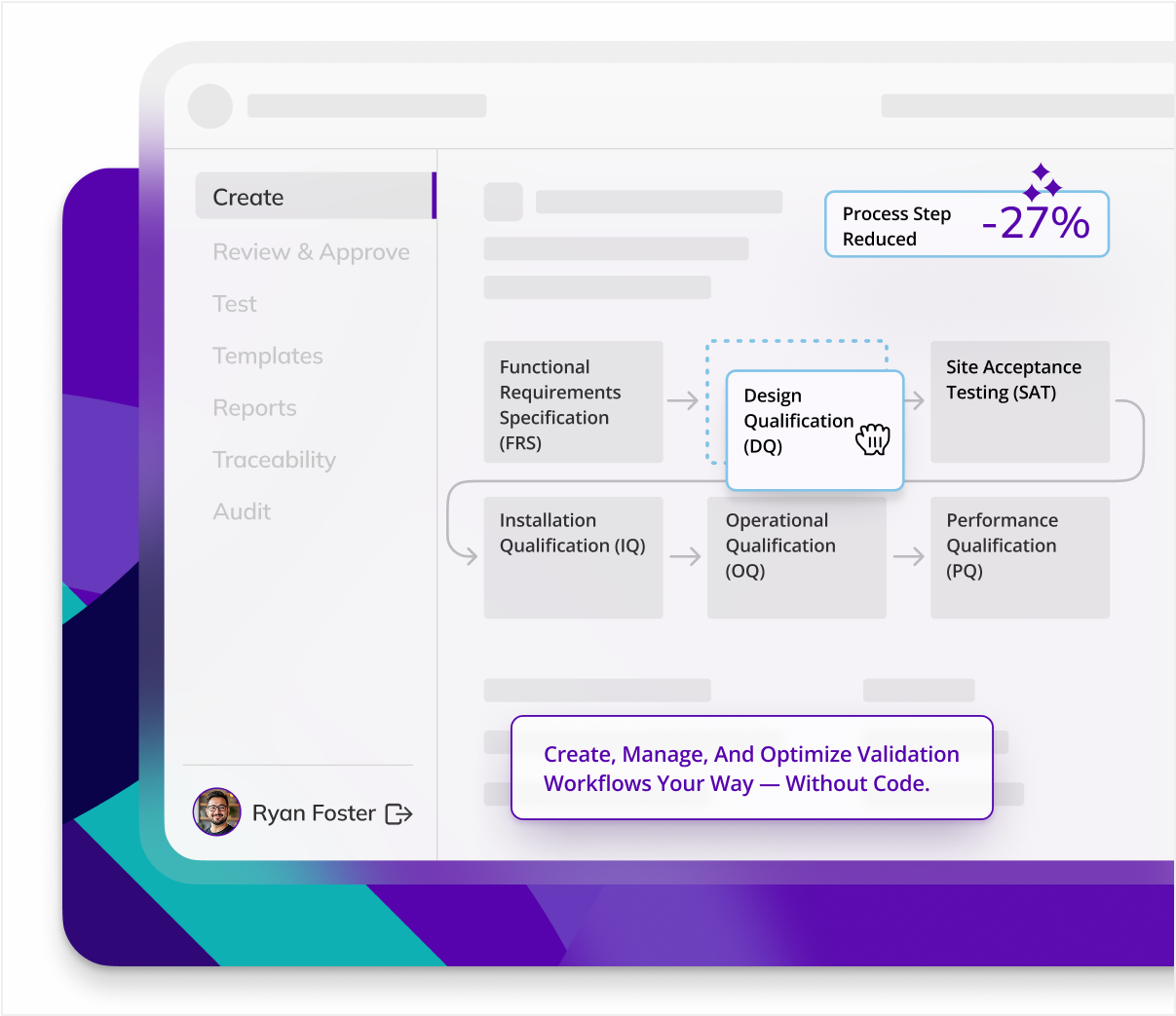
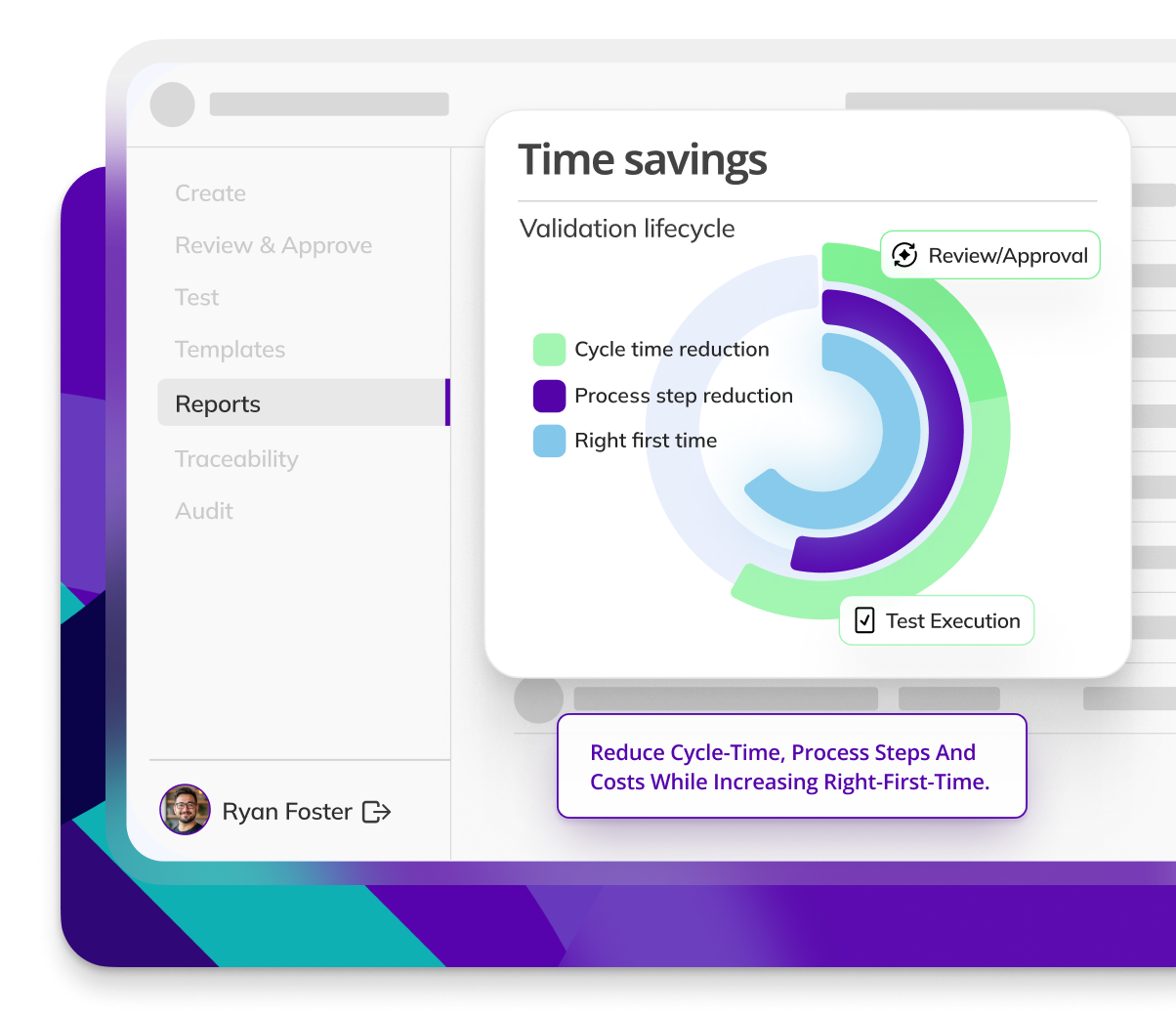
Kneat is proven to reduce cycle time by an average of 50% or more in multiple customer authored case studies.
CSAT Score
97% of our customers rate our customer support as either ‘Very Good’ or ‘Excellent.’
PLAN TO RENEW
Customer feedback via industry analyst SoftwareReviews.
Revolutionize your validation
Digitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Book a demo