Process validation is crucial in guaranteeing the excellence, safety, and dependability of products and services across various industries. At its essence, process validation represents a systematic and well-documented approach to showcasing that a given process consistently generates outcomes aligning with predetermined specifications and established quality benchmarks.
Whether in the realms of pharmaceuticals, medical device production, food processing, automotive manufacturing, electronics, consumer packaged goods, or any other sector subject to stringent regulations, the significance of process validation cannot be overstated. It serves as an indispensable component for upholding and enhancing product quality, safety, and the overall prosperity of an organization.
What Is process validation and why does it matter?
Process validation is the set of activities executed to establish scientific evidence affirming the consistent capability of a process to deliver products of high quality. It is characterized by the gathering and examination of data, spanning from the initial process design phase to full-scale commercial production.
Within the realm of life sciences manufacturing, process validation is the linchpin for safeguarding patient safety, maintaining uniform product quality and efficacy, and fostering cost efficiency, waste reduction, market competitiveness, and compliance with regulatory standards. Without process validation, the life sciences industry would lack the necessary mechanisms to produce products that are safe, effective, and reliable. This is why process validation is not just a best practice but an absolute necessity.
Safeguarding patient safety
The main objective in manufacturing is to create products that are safe and effective for patients. A faulty manufacturing process has the potential to introduce irregularities or flaws in the end product, jeopardizing the safety of patients. Process validation functions as a protective measure against these risks by meticulously assessing and confirming each stage of the production process. This scrutiny guarantees that every product is reliable and secure for consumption.
Uniformity in product quality and efficacy
In the pharmaceutical realm, fluctuations in product quality can yield significant repercussions. Even slight deviations in the manufacturing process may result in disparities in the efficacy, safety, and overall quality of drugs. Process validation plays a pivotal role in instituting and upholding unwavering quality by pinpointing critical process parameters (CPPs) and guaranteeing they adhere to predefined limits. This steadfast consistency is essential for cultivating and preserving patient confidence.
Cost efficiency and waste reduction
Streamlined manufacturing processes go beyond quality assurance; they also involve cost optimization. Process validation can pinpoint potential areas of resource wastage or inefficiencies. Through the refinement of processes, companies can diminish waste, conserve resources, and enhance the cost-effectiveness of production.
Market competitiveness
In a constantly changing life sciences landscape, manufacturers with the ability to consistently provide top-notch products enjoy a competitive advantage. Process validation empowers companies to not only meet but surpass quality benchmarks, rendering their products more appealing to both consumers and healthcare providers.
Regulatory compliance
Life sciences manufacturing stands as one of the most rigorously regulated industries on a global scale. Regulatory bodies, including the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and various others, impose strict requirements to guarantee both product quality and patient safety.
Adherence to these regulations is not discretionary; it constitutes a legal obligation. Process validation serves as the means by which companies showcase their commitment to complying with these regulations.
The history of process validation
The concept of validation emerged in the late 1970s in response to significant quality issues within the pharmaceutical industry. Before this, the assessment of pharmaceutical quality relied on testing samples taken from the final product.
As industries expanded in complexity and global reach, the imperative for consistent product quality and safety transcended the pharmaceutical realm. Sectors such as food, automotive, and electronics recognized the crucial role of process validation in averting defects and ensuring the reliability of their products. The advent of quality management systems like Total Quality Management (TQM) and Six Sigma further underscored the significance of validation in the pursuit of excellence.
In the course of time, the idea of process validation has evolved into a more systematic and risk-based approach. Presently, it constitutes an integral facet of quality management, ensuring that products adhere to stringent standards while simultaneously propelling continuous improvement and innovation across diverse industries.
Process validation terms to know
Here’s a summary of the 12 key requirements for process validation as outlined in the FDA Guidance for Industry: Process Validation: General Principles and Practices:
1) Process understanding: Manufacturers should have a deep understanding of their processes, including critical process parameters and critical quality attributes. This involves characterizing the process, determining potential variability points, and assessing the impact to the product when variability transpires.
2) Lifecycle approach: Process validation should be viewed as a lifecycle approach, covering three stages: Process Design, Process Qualification, and Continued Process Verification (CPV).
3) Risk-based approach:: A risk-based approach should be employed to prioritize critical process parameters and quality attributes. This involves identifying potential risks to product quality and patient safety during each step of the process as well as the controls in place to mitigate the known risks.
4) Quality systems:: Firms are expected to implement a robust pharmaceutical quality system to manage and control their manufacturing processes effectively.
5) Validation master plan: Manufacturers should develop a Validation Master Plan (VMP) that outlines the overall strategy for process validation, including the rationale for choosing specific validation approaches.
6) Validation protocols: Detailed validation protocols should be established, including clear predetermined objectives, testing methods, acceptance criteria, and responsibilities.
7) Change control: There should be a robust change control system in place to manage any changes to validated processes. Significant changes may require revalidation.
8) Continued process verification (CPV): CPV should be implemented, involving ongoing monitoring and analysis of process data. CPV ensures that processes remain in a state of control.
9) Data integrity: Data integrity is critical throughout the validation process, and manufacturers should have appropriate procedures and controls to maintain data accuracy, reliability, and completeness.
10) Documentation: Thorough documentation is essential. It should include comprehensive records of all aspects of the validation process, including master batch records, protocols, reports, and sampling worksheets.
11) Quality by design (QbD): The FDA encourages a Quality by Design (QbD) approach, which emphasizes the design of quality into the product and process from the outset.
12) Statistical methods: The use of statistical methods in data analysis and process control is recommended for process validation, helping ensure the robustness and reliability of processes.
Analyzing process validation methodologies
Process validation activities can be described in three stages.
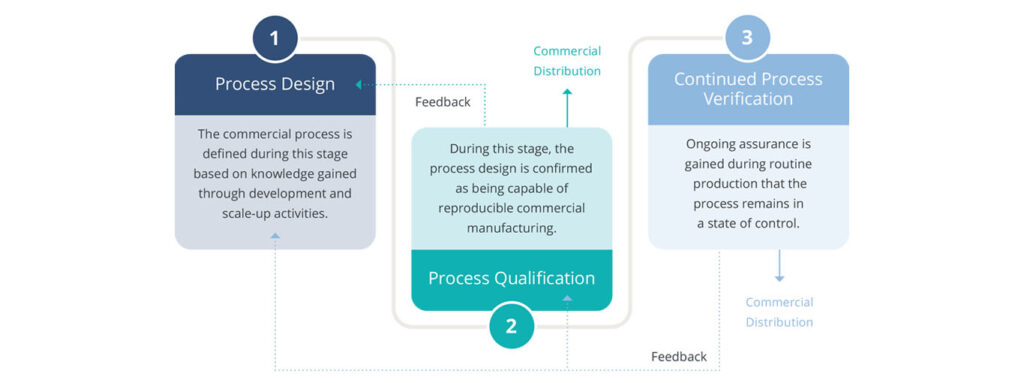
- Stage 1 – Process design: The commercial process is defined during this stage based on knowledge gained through development and scale-up activities.
- Stage 2 – Process qualification: During this stage, the process design is confirmed as being capable of reproducible commercial manufacturing.
- Stage 3 – Continued process verification (CPV): Ongoing assurance is gained during routine production that the process remains in a state of control.
Process design
Process validation begins with the initial phase, which is process design. In this stage, manufacturers define and create a detailed plan for how a drug or biologic product will be manufactured. The objectives include:
- Identifying critical quality attributes (CQAs): These are the characteristics of a product that are essential to meet its desired quality standards. For example, the potency of an active pharmaceutical ingredient (API), product stability, and sterility are CQAs.
- Defining critical process parameters (CPPs): These are the variables in the manufacturing process that can affect the CQAs. Identifying and controlling CPPs is vital to ensuring product consistency and quality. For instance, temperature, pressure, and mixing duration might be critical process parameters in a tablet manufacturing process.
- Developing a detailed process flow: Manufacturers create a step-by-step blueprint of the manufacturing process, including equipment, materials, and operating procedures which will be encompassed in a Master Batch Record (MBR).
- Drafting a validation master plan: This document outlines the overall strategy for process validation, including the scope, resources, responsibilities, and timelines.
Process qualification
The second stage of the process validation lifecycle is process qualification. It involves executing the validated process as per the design and ensuring that it consistently delivers products meeting the predefined quality attributes and process parameters. Key components of process qualification may include:
- Installation qualification (IQ): This verifies that equipment and systems are correctly installed, ensuring that they meet specifications.
- Operational qualification (OQ): This confirms that equipment operates according to predetermined specifications and settings, and it can produce the desired results.
- Process performance qualification (PPQ): In this step, the process is tested to ensure that it consistently produces products meeting the predefined quality attributes. This typically involves multiple runs to establish a track record of consistent performance.
Continued process verification
The third and final stage of the process validation lifecycle is continued process verification (CPV). Unlike the previous stages, CPV is an ongoing process that extends beyond the initial qualification. Its objectives include:
- Continuous monitoring of the manufacturing process: This involves real-time data collection and analysis to detect deviations and trends that may affect product quality.
- Data-driven decision-making: CPV relies on statistical tools and analysis to make informed decisions about process control and improvements.
- Risk management: By identifying and mitigating risks in real-time, CPV ensures that the manufacturing process remains in a state of control.
These three stages represent a comprehensive approach to ensuring product quality, safety, and efficacy from the design and development phase through commercialization and the product’s entire lifecycle. They enable manufacturers to adapt to evolving requirements and challenges, maintain consistent quality, and respond to emerging issues in real-time, ensuring that patients receive safe and effective medicines.
Emerging trends and innovations in process validation
Process validation is evolving rapidly, driven by technological advancements. Key trends and innovations in process validation include:
- Process analytical technologies (PAT)
- Digitalizing process validation
Process analytical technologies (PAT)
Process Analytical Technologies (PAT) is a transformative approach that leverages advanced analytical tools and technologies to monitor, analyze, and control pharmaceutical manufacturing processes in real time.
It represents a paradigm shift from traditional, batch-oriented quality control to a continuous and dynamic monitoring approach. The key roles of PAT in real-time process management are as follows:
- Real-time data collection: PAT encompasses various analytical techniques, including spectroscopy, chromatography, and in-line sensors, that enable continuous process verification and the collection of data during the manufacturing process. These technologies provide immediate insights into critical process parameters, quality attributes, and deviations.
- Early detection of deviations: One of the primary advantages of PAT is its ability to detect deviations from established process parameters in real time. By comparing real-time data to predefined control limits, PAT can identify issues as they occur, allowing for timely corrective actions.
- Rapid decision-making: With the continuous stream of data provided by PAT, pharmaceutical manufacturers can make informed decisions in real time. This ability to act swiftly is vital in preventing quality issues, minimizing waste, and optimizing resource utilization.
- Automation and control: PAT technologies can be integrated with process control systems, enabling automated adjustments when deviations are detected. This level of automation ensures that the process remains within defined boundaries.
Digitalizing process validation
The digitalization of the validation lifecycle involves the integration of advanced digital technologies into the validation process. This includes the use of digital platforms, automation, and cloud-based solutions to streamline data collection, workflows, management, and analysis.
Benefits of using a fully digital validation system such as Kneat Gx for process validation include:
- Audit readiness: Digital validation platforms enhance audit readiness by providing real-time data access, traceability, and automated compliance checks, streamlining record-keeping, and ensuring regulatory compliance for a seamless audit process.
- Automation: User defined templates automate the development of validation documentation packs.
- Configurability: An intuitive user interface can result in fast deployment and user adoption. Easy configurability means you map your process on the digital validation platform.
- Dashboard reporting: Run status reports or create progress reports using detailed filters. Digitalization enables real-time visibility, providing instant insights into the status of the manufacturing process at macro and micro levels. This allows for immediate corrective actions when deviations are detected.
- Data integrity: Enables 21 CFR Part 11/Annex 11 compliance with ALCOA+ principles.
- Data security: Robust cybersecurity protects sensitive data, ensuring compliance with regulatory standards.
- Document management: All your document files such as MS Word, Excel, PDF, images, and more, may be stored, controlled, reviewed, and approved within a digital validation system.
- Drawing management: Engineering drawings, including live walkdown markups, may be stored, controlled, reviewed, and approved.
- Enhanced efficiency: Digital tools reduce manual paperwork, streamline communication, and improve the overall efficiency of the validation process.
- Electronic logbooks: Used for compliant data capture in laboratories or on the manufacturing floor.
- Integration APIs: Integration with other key software systems such as PAT, to capture and support data.
- Online test execution: Executing testing digitally on your device, complete with integrated deviation lifecycle management and all test evidence.
- Remote review and approval: Secure cloud-based technology enables globally remote review and approval, in parallel or in sequence, so you can work from anywhere online.
- SaaS: Software-as-a-service delivered on a secure AWS cloud infrastructure, complete with elastic scalability, high availability, and zero downtime.
Removing paper from validation processes makes documents dramatically more accessible, lowers overhead, reduces cycle times, and improves accuracy and efficiency of all validation processes.
Futureproof your process validation with Kneat Gx
Digital validation is about more than removing paper from the process, it’s a move towards Pharma 4.0™, leveraging data, connecting sites, machines, and professionals to enhance product quality.
Our digital validation platform Kneat Gx is purpose-built for highly regulated companies, bringing efficiency and transparency to process validation with a focus on 21 CFR 11/Annex 11 compliance.
Kneat Gx is trusted by eight of the world’s top 10 pharmaceutical manufacturing companies, to manage the entire lifecycle of their validation processes.
The best way to know if Kneat Gx is right for you is to book a demo. You’ll receive a one-on-one demonstration of Kneat Gx, and an expert will be on hand to answer all your questions.






