Process validation is a critical aspect of ensuring product quality and safety in various industries, including pharmaceuticals, medical devices, and biotechnology. It involves systematically establishing evidence that a process consistently produces a product meeting predefined specifications and quality attributes.
While the concept of process validation is straightforward, its execution can be quite challenging. As industries strive for higher standards, regulatory agencies demand greater rigor, and technologies continue to advance, traditional methods of process validation face increasing challenges. The solution to many of these challenges lies in embracing the digital transformation of process validation.
Learn more about the evolution of process validation, the challenges and opportunities it presents, and the benefits of adopting a fully digital process-centric and data-driven approach in our Digital Process Validation Guide.
This article explores some of the key challenges in process validation and provides valuable strategies to overcome them, ensuring a smoother journey towards achieving compliance and efficiency.
The Significance of Process Validation
Process validation is not just a regulatory requirement; it is a fundamental quality assurance step that ensures products consistently meet the desired specifications. It plays a vital role in risk management, as the failure to validate processes can lead to variations in product quality, safety concerns, increased costs, and, in some cases, regulatory penalties.
Understanding the Challenges
- Complexity of Processes: In many industries, especially pharmaceuticals and biotechnology, processes are intricate and involve numerous variables. Managing and validating such complex processes can be overwhelming.
- Changing Regulatory Requirements: Regulatory standards are continually evolving, making it difficult to keep up with the latest requirements and ensuring compliance.
- Data Management: Collecting, analyzing, and managing the vast amount of data generated during validation can be a logistical nightmare.
- Interdepartmental Collaboration: Process validation often requires close collaboration between different departments, and this can be challenging due to differences in priorities and communication barriers.
- Resource Constraints: Adequate resources, both in terms of human resources and budget, are often required for successful process validation. Many organizations may face limitations in this regard.
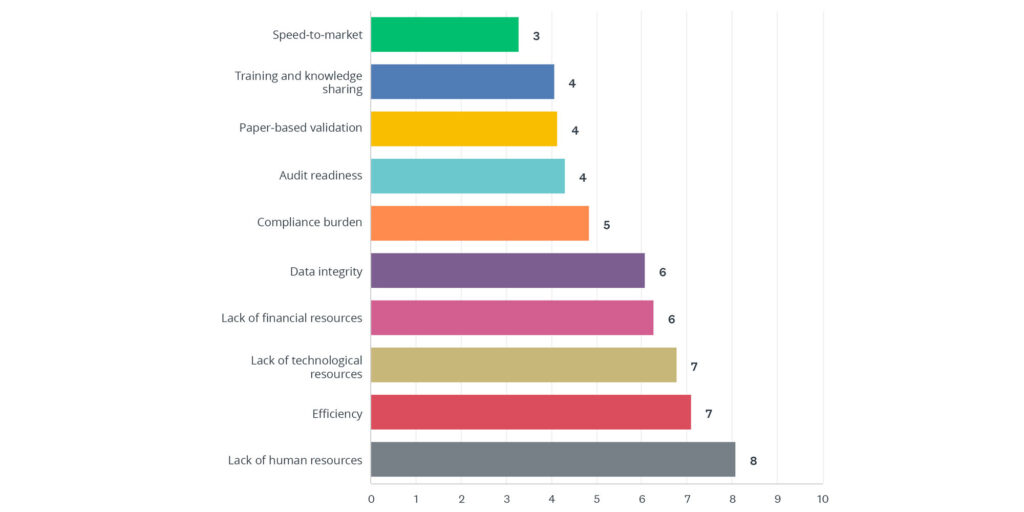
According to the State of Validation 2023 report, “Lack of Human Resources” was ranked as the number one validation-related challenge facing organizations, with 39 percent of all respondents ranking this as the top challenge, followed by “Efficiency”, and a “Lack of Technological Resources” rounding out the top-three.
To uncover more validation trends, challenges, and opportunities and see how your organization’s validation program measures up, read the full report.
Overcoming Process Validation Challenges
1) Risk-Based Approach: Implementing a risk-based approach to process validation can help prioritize efforts where they are most needed. Identifying critical process parameters and focusing validation efforts on them can simplify the process.
2) Clear Documentation and Procedures: Comprehensive documentation and standardized procedures are essential to ensure that the validation process is consistent and transparent. Clear guidelines and documentation help teams follow the process efficiently and adhere to regulatory requirements.
3) Regular Training: Investing in training and development for staff members is crucial. This includes staying up to date with changing regulations and standards, as well as enhancing skills related to data analysis and validation techniques.
4) Advanced Data Management Tools: Implementing advanced data management and analytics tools can streamline the process of collecting and analyzing validation data. These tools can help identify trends, outliers, and potential issues more efficiently.
5) Interdepartmental Communication: Encouraging effective communication and collaboration among different departments is essential. Establishing cross-functional teams that meet regularly to discuss validation progress and challenges can bridge the communication gap.
6) Validation Master Plan: Develop a comprehensive Validation Master Plan that outlines the scope, objectives, and responsibilities of the validation process. This plan serves as a roadmap for the entire validation effort, ensuring that everyone is on the same page.
7) Supplier Collaboration: In industries that rely on a supply chain, collaborating with suppliers can be beneficial. Ensuring that suppliers meet specific quality standards and validate their processes can reduce the risks associated with raw materials.
8) Continuous Monitoring and Review: Post-validation, it’s crucial to continuously monitor the process to ensure it remains in a state of control. Periodic reviews and audits help identify deviations and initiate corrective actions promptly.
9) Validation Software: Utilize specialized validation software solutions
to streamline validation and document management and facilitate compliance with industry standards.
10) Process Automation: Implement automation wherever possible in the validation process. Automation can significantly reduce the potential for human error and expedite data collection and analysis.
Final Thoughts
In a rapidly evolving regulatory landscape, the ability to adapt and innovate in process validation is key to long-term success and sustainability in various industries.
Process validation is a multifaceted challenge that, when successfully navigated, can lead to improved product quality, safety, and compliance. By understanding the complexities and risks associated with process validation, and by adopting the strategies outlined in this article, organizations can overcome these challenges and ensure that their processes consistently produce high-quality products.
Learn More in the Digital Process Validation Guide
Take your validation expertise to the next level with our Digital Process Validation Guide. Download your copy and learn more about the evolution of process validation, the challenges and opportunities it presents, and the benefits of adopting a fully digital process-centric and data-driven approach.




