In the dynamic world of pharmaceuticals, biotechnology, and medical devices, validation remains a cornerstone of ensuring product quality, safety, and compliance. As we move toward 2024, it’s essential to keep a keen eye on emerging trends that are shaping the validation landscape. In this article, we will delve into three pivotal trends that are poised to drive validation practices in the coming year—Computer Software Assurance (CSA), GAMP 5®, and Validation 4.0.
1. CSA: Efficient Risk-Based Approach to CSV
The traditional approach to Computer System Validation (CSV) has been in use since the introduction of FDA 21 CFR Part 11, 1997. Since then, CSV has often been described by those working in the validation and quality assurance industry as burdensome, due to the need for large paper-based documentation packs with significant volumes of screenshot or printout attachments.
The FDA’s draft guidance on Computer Software Assurance for Production and Quality System Software (CSA), released in September 2022, introduced a new discussion to the industry. It introduced a paradigm shift in how computerized systems can be validated and maintained. While traditional CSV focuses on ensuring that systems meet predetermined requirements, CSA emphasizes a risk-based approach that adapts validation efforts to the potential impact on product quality and patient safety.
Validation processes are structured to efficiently test vital components. By eliminating redundant tasks and excessive administrative procedures, a risk-centric method is employed to guarantee testing prioritizes functions or procedures that present a moderate to high risk to patient safety, product quality, or data integrity. Less emphasis is placed on examining lower-risk system features, resulting in a substantial reduction of paperwork through informal and spontaneous testing procedures.
Our eBook, Understanding Computer Software Assurance will give you a foundation of understanding so you can take the first step in your CSA journey. You’ll learn:
- What CSA is and why it is recommended by the FDA
- How CSA benefits life sciences companies
- Why digital validation complements CSA
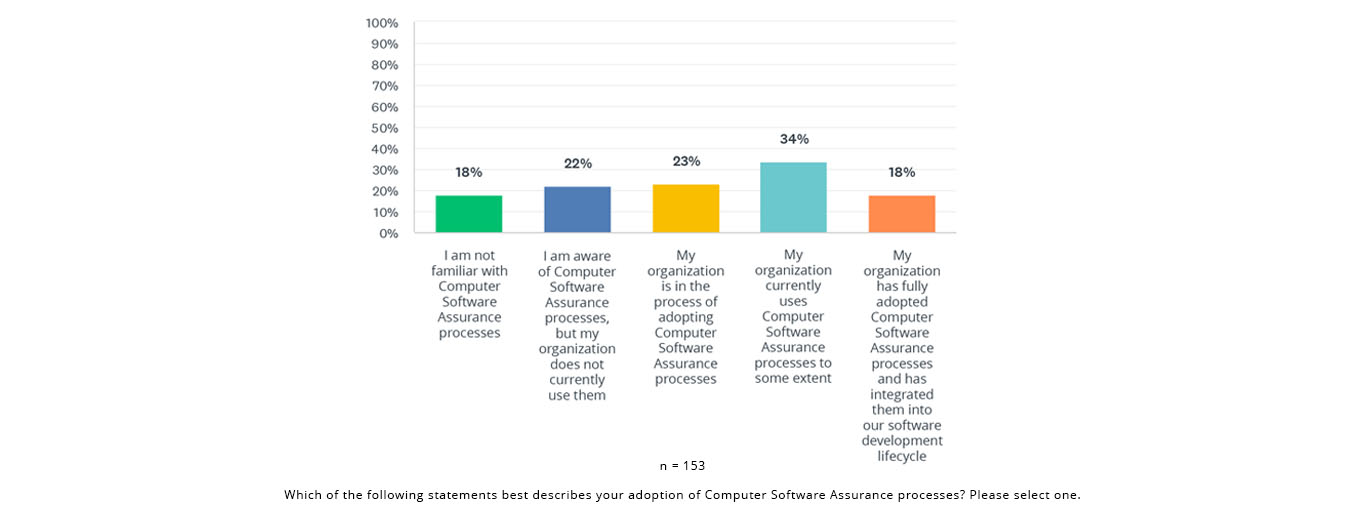
The State of Validation 2023 survey found that a majority (57%) either use or are adopting CSA processes: 34% indicated that their organization currently uses CSA processes to some extent; an additional 23% reported that their organization is in the process of adopting CSA processes, suggesting a growing trend toward adopting this approach.
As more and more organizations start to adopt the CSA approach, companies should hold vendors responsible for testing and ensuring their software’s risks and efficacy.
The trend towards CSA has far-reaching implications for validation teams. As organizations adopt CSA principles, they can expect improved efficiency, reduced validation timelines, and increased agility in responding to evolving requirements.
Watch our on-demand webinar with CSV specialist Darren Geaney “Achieving Computer Software Assurance with Digital Validation” to learn more about what the FDA’s guidance means for validating computer systems.

2. GAMP 5: Underpinning CSA Guidance and Beyond
The International Society for Pharmaceutical Engineering (ISPE)’s Good Automated Manufacturing Practice (GAMP) framework has long been a guiding light for validation professionals. With the release of the second edition of GAMP 5, the framework aligns seamlessly with the principles of CSA. GAMP 5 emphasizes the importance of a risk-based approach and provides guidance on integrating automated testing, continuous validation, and real-time monitoring into software development and maintenance processes.
GAMP 5’s alignment with CSA guidance provides validation practitioners with a comprehensive roadmap for embracing modern validation practices. The framework advocates for the use of standardized approaches, risk assessment methodologies, and effective documentation to ensure that computerized systems are validated appropriately and remain in a validated state throughout their lifecycle.
Furthermore, GAMP 5 goes beyond CSA and addresses the challenges posed by emerging technologies such as artificial intelligence, machine learning, and the Internet of Things. It offers insights into how these technologies can be validated while maintaining compliance with regulatory requirements. As the industry moves towards digital transformation, GAMP 5 serves as a vital tool in fostering a structured and standardized approach to validation that is adaptable to new technologies and methodologies.
3. Fostering Validation 4.0: Embracing Innovation and Efficiency
Validation 4.0 represents the convergence of cutting-edge technologies with validation practices. It leverages the power of Industry 4.0 principles, such as automation, data exchange, and artificial intelligence, to transform validation into a more efficient, agile, and predictive process. Validation 4.0 envisions a future where validation is not merely a compliance exercise but an integral part of the product lifecycle, contributing to operational excellence and improved patient outcomes.
Data Analytics and Machine Learning
One of the key pillars of Validation 4.0 is the utilization of data analytics and machine learning to make informed decisions. By analyzing vast amounts of validation data, organizations can identify patterns, anomalies, and optimization opportunities. Predictive modeling enables proactive risk management and enhances decision-making, ensuring that validation efforts are focused where they matter the most.
Automation
Automation plays a pivotal role in Validation 4.0. Automated testing, continuous validation, and the use of digital twins for virtual validation are becoming standard practices. These technologies not only expedite the validation process but also reduce human error and enable scalability. Automated validation tools enable organizations to achieve consistent and reproducible results, even in complex and diverse product portfolios.
Validation 4.0 emphasizes collaboration and knowledge sharing. Cloud-based platforms like Kneat and collaborative tools facilitate real-time communication and data sharing among validation teams, developers, and other stakeholders. This collaborative approach streamlines communication, accelerates decision-making, and ensures that all parties are aligned in terms of validation goals and requirements.
Final Thoughts
The validation landscape is evolving at a rapid pace, driven by regulatory shifts, technological advancements, and the need for efficiency and innovation. The FDA’s CSA guidance, bolstered by GAMP 5, is reshaping how validation is approached, emphasizing risk-based, continuous validation practices. Validation 4.0 is propelling the industry towards a future where data-driven decision-making, automation, and collaboration drive validation excellence.
As we move toward 2024, organizations that embrace these trends will not only meet regulatory requirements but also position themselves as pioneers in delivering high-quality, safe, and compliant products to the market.

Learn More About CSA
Watch our on-demand webinar “Futureproofing Your CSV Program” to discover more about CSV landscape.
You’ll also learn:
- What the FDA’s September 2022 CSA guidance means for validating computer systems
- Efficiencies and assurances of performing CSA using a digital validation system
- How in-vitro diagnostics industry leader Fujirebio Diagnostics, Inc. (FDI) uses Kneat to provide CSV at scale







