Technological advances in the life sciences as part of the Pharma 4.0 industry evolution have accelerated rapidly in recent years, resulting in increased demand for innovative, non-automated solutions that enable quick and easy digital Computer System Validation (CSV).
Since the German government introduced the industry 4.0 initiative in 2011, the International Society for Pharmaceutical Engineering (ISPE®) has been championing and driving Pharma 4.0™ within the life sciences sector. As such, the ability for all validation, quality, and compliance professionals to provide efficient CSV services has continued to grow in importance over the last 11 years—as the volume of computerized solutions used in the manufacturing process increases.
In this article we examine the uptake of Pharma 4.0 and highlight:
- Its history
- Its goals
- Industry concerns
- Its impact on CSV
- Challenges of using automated CSV tools
- CSV opportunities and trends
The history of Pharma 4.0
Pharma 4.0 is a derivative of a broader manufacturing initiative known as Industry 4.0 (1), started by the German government in 2011 as part of their high-tech strategy to promote the computerization of industry. Industry 4.0 is defined by the presence of ‘smart’ manufacturing technologies which produce and interpret data to create intelligence—achieving both real-time and predictive capabilities to optimize performance.
Within the life sciences sector, Industry 4.0 is being progressed by the International Society for Pharmaceutical Engineering (ISPE) (2) who is developing a roadmap to introduce this initiative to the industry as Pharma 4.0. It’s Pharma 4.0 Special Interest Group (SIG), whose members are all industry experts, has developed a model to apply the principles of Industry 4.0 to Pharma 4.0, in order to accelerate the transformation of existing facilities to a Pharma 4.0 standard.
Operating Model: From Industry 4.0 to Pharma 4.0
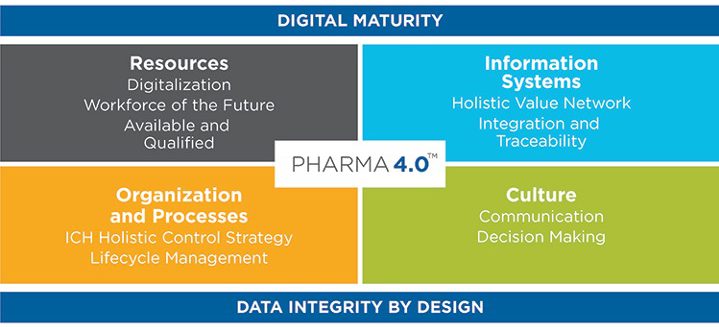
Image source © ISPE®
Goals of Pharma 4.0
The primary goal of Pharma 4.0 is to digitalize the life sciences industry and by doing so, bring it more fully in line with the concept of the smart factory, and other industries. According to the ISPE’s website, its objective is, “To enable organizations involved in the product life cycle to leverage the full potential of digitalization to provide faster innovations for the benefit of patients.” (3)
The initiative aims to bring production facilities to a new level of speed and accuracy and will integrate innovative machine intelligence and automated technologies into workflows.
Industry concerns
Although the life sciences industry is highly competitive and fluid, the sector has been slower to utilize innovative technology when compared to other industries. (4) While the life sciences industry does share many practices and procedures with other industrial sectors, it must also consider some highly complex product portfolios, critical product life cycles, and risk to patient safety.
Industry concerns about speed, safety, and quality, coupled with high regulation and long-lasting subsequent change management processes have all historically contributed to a slower pace of uptake by life sciences organizations, though this outlook has shifted significantly in recent years.
Discover and apply validation technologies, regulations, and best practices at VALIDATE 2022 — an annual validation experience, powered by Kneat, that gathers validation and quality professionals from around the world in Boston on November 16-17.
Learn from 16 expert-led sessions and connect with your peers at VALIDATE’s offsite social experiences.

The impact of Pharma 4.0 on CSV
At the heart of any technological change in any highly regulated industry is Computer Systems Validation (CSV). Within the Life Sciences, industry regulations require that all critical computer systems used for the manufacturing, storage, or testing of a drug product be validated and maintained in a validated state throughout the computer system’s life cycle. Failure to demonstrate a state of control can result in regulatory citations, fines, product recall and risk to product license. In the United States, such changes are governed by the FDA’s 21 CFR Part 11.10(a) (5) regulation, with EU Annex 11, section 4 (6) mandating in the European Union.
The regulations define key areas of system validation and data management that the regulator views as having the highest likelihood of failure, leading to data misappropriation. The primary goal of these regulations is to foster safe, validated, computer systems for research, clinical study, maintenance, manufacturing, and distribution of medical products.
Installing and maintaining computer systems in regulated environments requires a structured life-cycle approach, from vendor selection to configuration and acceptance testing through to go-live and change management (patching, upgrading, and configuration change). As life sciences manufacturers seek efficiency in manufacturing processes through computer systems, demand to install and maintain an array of increasingly complex computer systems and software grows—as does their risk profile.
Challenges of automated CSV tools
Automated CSV tools, like Micro Focus Unified Functional Testing (UFT), formerly known as QuickTest Professional, and TestDirector, typically place the burden of set up on validation teams. As such, automated CSV solutions are arguably better suited to software vendors or very large enterprise companies with huge validation teams and resources to support.
Resource availability
For smaller, more agile, teams, the onus of configuration can create big hurdles—such as resource availability to author, run, and maintain automated scripts—which require writing change management documents, code locks, etc., and necessitate significant coding skills. The additional work involved when utilizing automated tools can be challenging and time consuming for more compact teams who must add these processes to their workflow without impacting normal everyday CSV tasks and slowing down business deliverables.
Consistency of test data and ability to rewrite quickly
Despite these factors, the biggest hurdle caused by automated CSV tools for all validation teams working in the life sciences industry, regardless of size, is often the requirement to maintain a consistent set of test data—and then the ability to rewrite over that data set quickly to run the same testing again. The use of automated solutions, like UFT, forces reliance on the same records to be there every time in the same exact state, as those values get written into the code.
This is much easier for a vendor since they are testing the same software over and over. It makes perfect sense that a vendor can run regression testing on the same data set that it used three versions ago. However, for a life sciences company, which typically has upwards of 30 different business systems to test throughout the year, this is not feasible or practical. The best-case scenario is probably to test with a current copy of Production, and that copy today is quite different from the copy pulled previously. When a data set changes, the code in the automated scripts must also change, resulting in a requirement to update test-scripts every time the validation team needs to run them.
CSV opportunities and trends
Technological advances in the life sciences industry have accelerated rapidly in recent years, driven in part by thought leaders like the ISPE whose Pharma 4.0 initiative has resulted in an increased demand for digital solutions. No-code CSV solutions, like Kneat Gx—our paperless Computer System Validation and Verification application —enables validation teams to author and execute in the traditional manner, quickly and easily without paper and produce faster speed to market times and better outcomes for businesses.
Kneat Gx was designed by our software engineering team to be used by all validation and quality professionals, removing the requirement to have any coding or knowledge of programming languages. Our application is part of the “self-service” software trend that empowers users to create, manipulate, and employ data-driven applications to do their work better, faster, and more efficiently. Today eight of the top ten global pharmaceutical companies use our no-code digital validation software to find efficiencies in their validation processes.
Other benefits of using Kneat Gx for CSV include:
- Facilitates best practice risk-based, lean, life-cycle approach to validation
- Eliminates 100% of paper-records
- Paperless test execution with integrated deviation management
- Productivity improvement of up to 100%* and cycle time savings of up to 50%*
- 21 CFR Part 11/EudraLex Annex 11 compliant
- Standardizes CSV processes for all project and IT system life-cycle management
- ‘ALCOA’ Data Integrity best-practice
- Instantly see why approved data was changed, when and by who
- Macro and micro visibility into all aspects of the process in real time
- Protocol GDP errors minimized
- System status monitoring – validated, review due/complete, etc.
- Audit ready in real time

Fujirebio Diagnostics, Inc., uses Kneat to provide CSV at scale
In-vitro diagnostics industry leader Fujirebio Diagnostics, Inc. (FDI), (7) selected Kneat to digitize its global CSV process with the aim of enabling its limited IT Compliance Team to deliver a large volume of complex computer-system projects, including the implementation and re-validation of systems at multiple manufacturing sites across five divisions.
As a result of this digital validation initiative, FDI achieved a 53% reduction in time for quality management (CAPA) system change control projects, reduced the number of working days for script execution from 15 to just seven on average, and eliminated reliance on a time-consuming ‘hybrid’ validation system, driving efficiencies for the validation team.
Read more about FDI’s story and why they chose Kneat.
References
- What is Industry 4.0, [https://www.forbes.com/sites/bernardmarr/2018/09/02/what-is-industry-4-0-heres-a-super-easy-explanation-for-anyone/?sh=14dff74c9788].
- ISPE, [https://ispe.org/].
- ISPE Pharma 4.0, [https://ispe.org/initiatives/pharma-4.0].
- ISPE Measuring Pharma’s Adoption of Industry 4.0, [https://ispe.org/pharmaceutical-engineering/january-february-2022/measuring-pharmas-adoption-industry-40].
- CFR – Code of Federal Regulations Title 21, [https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=11].
- EU Annes 11, [https://ec.europa.eu/health/system/files/2016-11/annex11_01-2011_en_0.pdf].
- Fujirebio Diagnostics, Inc. (FDI), [https://www.fujirebio.com/en].




