This year’s VALIDATE conference took place on May 15 and 16 in Berlin, showcasing the very latest innovations and advancements in validation technologies. The event centered around the theme “Digitalization and Change,” drawing inspiration from the Validation 4.0 initiative.
VALIDATE 2024’s focus was on addressing the critical challenge of leading organizations through the transition to a digital future. Attendees were empowered with the knowledge and tools needed to understand and implement the validation technologies and operational strategies essential for successful digitalization.
Take a look at some of the highlights from VALIDATE 2024, where AI, change management, Validation 4.0, and more took center stage.
Kneat Customer Morning
Kneat’s CEO, Eddie Ryan, opened the conference by affirming Kneat’s commitment to sharing knowledge on the digitalization of validation. He emphasized the goal of delivering impactful outcomes for the industry, setting a collaborative and forward-thinking tone for the event.

Day one of VALIDATE 2024 kicked off with exclusive customer sessions focused on the future of AI and its transformative impact on validation processes.
Kneat Gx Product Roadmap: Keith Holmes & Ruairi McGarry, Kneat
This session discussed how AI-driven innovations are set to enhance regulatory compliance, operational efficiency, and product quality.
Customers were given an overview of Kneat’s latest release, Kneat Gx 9.2 and an exciting preview of upcoming features in Kneat Gx, showcasing advancements designed to deliver smarter, faster, and more accurate validation practices. Kneat Gx 9.2 enhancements include:
- Advanced Search: broader search coverage and flexible options, such as the use of Boolean operators (using “and” “or” “not” to specify search results) and the ability to see multiple results per document and see preview snippets.
- Audit Readiness: view only area for approved content in ‘Collections’ and auditor role and area in app.
- Real-Time Requirements Traceability Matrix (RTM): RTM updates in real time as changes are saved, making it easy to reference sections within Test Documents.
You can see all the latest features and functionality of Kneat Gx in action during our monthly demo webinar. Register now to book your spot!
Global Governance and Applications: Jamie O’Donnell & Sarah Grogan, Kneat
This VALIDATE 2024 session focused on best practices in scaling Kneat Gx (for both a top-down and site approach), adoption, harmonization of processes, governance, ease of decision making, and more.
| Top-Down Approach | Site Approach |
|---|---|
| Corporate Level Decisions • Process • Site | Site-Specific Decision • Site-Specific Process • Site-Specific Guidance |
| Centralized IT Support | Centralized IT Support or Site-Based Support |
| Centralized Governance • Folder Structure • Naming Convention | Site Governance |
| Site-Specific Items • Gap Assessment • Specific Test Cases • Folder Structures for Sites | Moving From Site-Specific to Global • Consider Generic Templates & Structures Easily Scaled • Numbering Conventions |
Best Practice in Scaling Kneat Gx
Key takeaways included insights on governance of digital validation systems from the 2024 State of Validation industry survey:
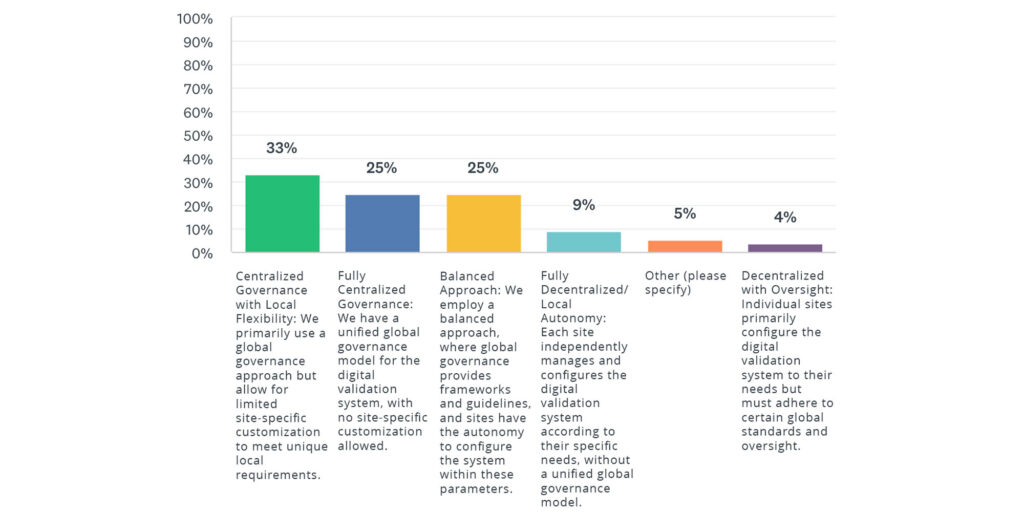
Most organizations favor centralized or balanced governance approaches for digital validation systems, indicating a need for standardization with local flexibility, while fewer organizations opt for fully decentralized governance due to concerns over consistency and compliance.
Discover the latest trends and insights in validation. Stay ahead in your industry with this comprehensive report on current best practices and future developments. Reserve your copy of the 2024 State of Validation Report .
Learning from Fellow Kneat Users
“The presentations at VALIDATE are really good, but one of the main reasons we attended the conference is to be able to connect with other Kneat users and learn about the experiences of how it has been implemented in other sites. We’re really trying to build those connections during our time here.”
Michael Ah-Cann, CSL Behring
Keynote
The keynote revealed how to unlock your full potential with a positive mindset and some new habits that make change an enjoyable daily experience.

Allister Frost outlined the five principles of his Future Ready Mindset:
- Discover how your current relationship with change could be limiting your potential and learn strategies to overcome these barriers.
- Consider innovative approaches to outpace competitors by thinking smarter and acting faster.
- Reflect on your aspirations for your future self and outline practical steps to become the person you aim to be.
- Utilize your inherent human superpowers to cement your irreplaceability at work.
- Embark on a journey to create the brilliant future you not only desire but truly deserve.
These sessions underscored Kneat’s commitment to staying at the forefront of digital transformation in highly regulated industries.
Audit Readiness and Harmonization & Globalization

The “Audit Readiness” session at VALIDATE 2024 was a comprehensive guide for professionals preparing for regulatory inspections. The session emphasized the importance of being ‘inspection ready,’ which means having systems and processes in place that are always prepared for an audit, ensuring compliance with regulatory standards.
A key highlight was the discussion on ALCOA+, a critical framework ensuring data integrity. ALCOA+ stands for Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Consistent, Enduring, and Available. This expanded version of the ALCOA principles underscores the need for data to be comprehensive and reliable throughout its lifecycle.
The session also covered the advantages of digital validation systems for maintaining audit readiness . Kneat Gx streamlines validation processes, enhances data accuracy, and ensures compliance through automated workflows and real-time monitoring.
Kneat Gx’s unique ‘Collections’ feature allows Kneat users to send approved documents to a dedicated staging area where non-standard users, such as auditors, can view and interact with the content. This allows for virtual “war rooms” or audit prep rooms, which streamline audits and make it easier for both regulatory bodies and you. With the expected rise of remote audits , this digital capability can provide time savings and reassurance that your company is audit ready.
Collections aren’t just for auditors, they’re also perfect for project review or other internal use cases.

The “Harmonization & Globalization” session at VALIDATE 2024 delved into the transformative impact of implementing digital validation systems in the pharmaceutical industry. Working with Kneat, Takeda and CSL Behring successfully enforced a standardized framework, ensuring new employees encounter a fresh system free from legacy documents. This consistency was further reinforced through global templates, facilitating a unified approach across different regions.
The key benefits of adopting a standardized digital validation system include resource sharing, increased efficiency, and language consistency. This creates a cohesive picture for regulatory inspections, making audits more comfortable and predictable. Best practices for maintaining compliance include simplifying processes to avoid duplicating documents across sites and utilizing change forms to track updates. This approach allows for effective trending of data, providing insights into the state of control within the company.
Education and training are vital for the successful implementation and adoption of digital validation systems. Looking ahead, the future of these systems in the pharmaceutical industry is promising, with potential advancements in AI and machine learning. These technologies could automate documentation processes and integrate regulatory requirements, ensuring ongoing alignment with global standards.
Innovation in Logbooks & Automation and Validation 4.0
Innovative Applications of Kneat Gx in Logbooks, Automation, & Beyond: Ian Harley & Alan Murphy, Alexion Pharmaceuticals Inc.
VALIDATE 2024 highlighted the transformative impact of Kneat Gx applications in Alexion’s Quality Control operations. Implementing an Electronic Logbook Management platform significantly reduced reliance on paper logbooks and forms, resulting in shorter testing and batch release lead times. The digitization improved data accessibility streamlined end-to-end data management, and mitigated quality and compliance risks.
Key quantitative benefits included reducing paper logbook issuance time from approximately two days to less than half a day and eliminating archival delays. Qualitative benefits encompassed enhanced reporting, analytics, searchability, and improved disaster recovery. This transition freed up QC capacity, shortened overall lead times, and aligned with sustainability goals by reducing paper usage.
Cultural and regional challenges were managed through standardized templates and comprehensive training programs. The adoption of Kneat Gx applications extended beyond QC to automation, enhancing review times and attachment accessibility while supporting ongoing audit readiness.
Alexion’s strategy focused on speed, agility, and enterprise value, achieving notable reductions in process lead times and inventory management, ultimately driving smarter decision capabilities and quality risk mitigation. This case study exemplified the significant benefits of integrating digital validation systems within pharmaceutical operations.

The VALIDATE 2024 session “Enablement of Pharma 4.0 & Validation 4.0” explored the integration of advanced digital frameworks in the pharmaceutical industry.
Pharma 4.0 focuses on leveraging digitalization, holistic value networks, and workforce transformation to enhance organizational processes and cultural communication. Validation 4.0 emphasizes continuous, real-time verification of product quality through digital means.
The session highlighted how AI can streamline documentation processes, from generating and reviewing documents to executing and verifying control measures. This digital shift enhances compliance, traceability, and decision-making, aligning with the industry’s evolving needs and regulatory standards.
Embracing Digitalization and Pharma 4.0
I’m really enjoying the conference as an attendee; it’s been really inspiring to hear the presentations this morning and really good to see other similar companies using Kneat to move into digitalization and Pharma 4.0.
Joe Bates, Cell & Gene Therapy, Catapult
Streamlining Engineering Solutions & Using Digitalization for Risk-Based CSV

At “Streamlining Engineering Solutions: Integrating Kneat Drawing Management at Novartis,” the focus was on the successful digital transformation of qualification processes.
Novartis implemented Kneat’s digital solution across multiple sites, significantly improving efficiency and data accessibility. Key milestones included the completion of a pilot, onboarding of sites, and integration with existing systems.
The integration of drawing management into Kneat enhanced Design Qualification processes, enabled lean approval workflows, and reduced costs. Challenges such as network issues and the migration of 40,000 drawings were addressed through collaborative efforts and user feedback, leading to a streamlined, efficient system that supports ongoing innovation and regulatory compliance.
Utilizing Digitalization for Risk-Based CSV: Darragh Boyle, Kneat
Computer Software Assurance (CSA), developed through collaboration between the FDA and industry, emphasizes a common-sense, risk-based approach to validation. It encourages scaling validation efforts based on risk assessment, focusing more resources on high-risk areas that directly impact patient safety and product quality. This method aligns with the principles of Pharma 4.0 , promoting improved quality, efficiency, and compliance.
Key success factors for CSA include leveraging vendor documentation, conducting risk-based evaluations, and scaling validation efforts accordingly. CSA can reduce unnecessary documentation by up to 80%, streamline processes through unscripted and ad-hoc testing, and reduce validation timelines by focusing on critical thinking and intended use.
Incorporating CSA into digital validation processes leads to significant benefits such as improved data integrity, reduced protocol generation errors, and more efficient use of resources.
The session concluded with key takeaways: leverage your vendors, take a risk-based approach, apply critical thinking, scale testing based on risk, and digitalize your validation processes for optimal outcomes in CSV.
Networking & Community Building
VALIDATE 2024 transcended the typical conference format, serving as a hub for fostering a robust, interconnected global validation community. With ample networking opportunities, such as social events around the city and on the River Spree, discussion groups, and interactive workshops, attendees were encouraged to share ideas, best practices, and forge lasting relationships.

Thank You
We extend our gratitude to all our speakers, customers, partners, and attendees for making VALIDATE 2024 an unforgettable experience. While Berlin provided the setting, it was the people, ideas, and connections that made this event truly exceptional.
A very special thank you to our Diamond sponsors, CAI , No deviation , and VEQTOR , and our Exhibitor sponsor, PiQup .
VALIDATE is a Must-Attend Event for Kneat Users
“I definitely recommend people come to VALIDATE. The discussions have been very interesting and it’s good to see the Kneat product, especially the developments in the platform.”
Lee Jones, Dechra Pharmaceuticals
Join Us in Boston at VALIDATE 2025
We eagerly anticipate the next VALIDATE conference in Boston, MA, where we expect even more advancements in validation and compliance, propelled by the innovative spirit and collaborative strength of today’s validation community.
Join us at VALIDATE 2025 in Boston on April 30 and May 1 and be at the forefront of validation innovation!







