As the use of technologies in the life sciences industry continues to evolve, the need for efficient and compliant Computer System Validation (CSV) remains a top priority. CSV ensures that systems used as part of the Production and as part of the Quality system meet regulatory standards, maintain data integrity, ensure product quality, and safeguard patient safety.
In 2024, several emerging trends are redefining CSV methodologies and tools, allowing organizations to improve efficiency, reduce costs, and enhance compliance. In this article, we explore some of these key trends, focusing on the ways technology and modern methodologies are transforming CSV processes.
1. Adopting a Risk-Based Strategy for Efficient Validation
The adoption of risk-based validation is transforming CSV by focusing resources on the most critical areas that impact product quality and patient safety. This approach, in line with the FDA’s Computer Software Assurance (CSA) framework, encourages companies to prioritize validation activities based on risk assessment, rather than treating all systems with equal scrutiny. By concentrating on high-risk areas, organizations can reduce the time and cost of validation while still ensuring robust compliance.
A risk-based strategy allows validation teams to make more strategic decisions and allocate resources effectively. For example, systems that pose minimal risk to patient safety may require less rigorous testing, while critical systems undergo more comprehensive validation. This targeted approach improves validation efficiency and ensures that companies meet regulatory requirements without unnecessary overhead.
According to the State of Validation study, the percentage of organizations in the process of adopting CSA has risen slightly from 23% in 2023 to 25% in 2024, indicating persistent interest (see graph below).
Trends in Adoption Rate of CSA Processes
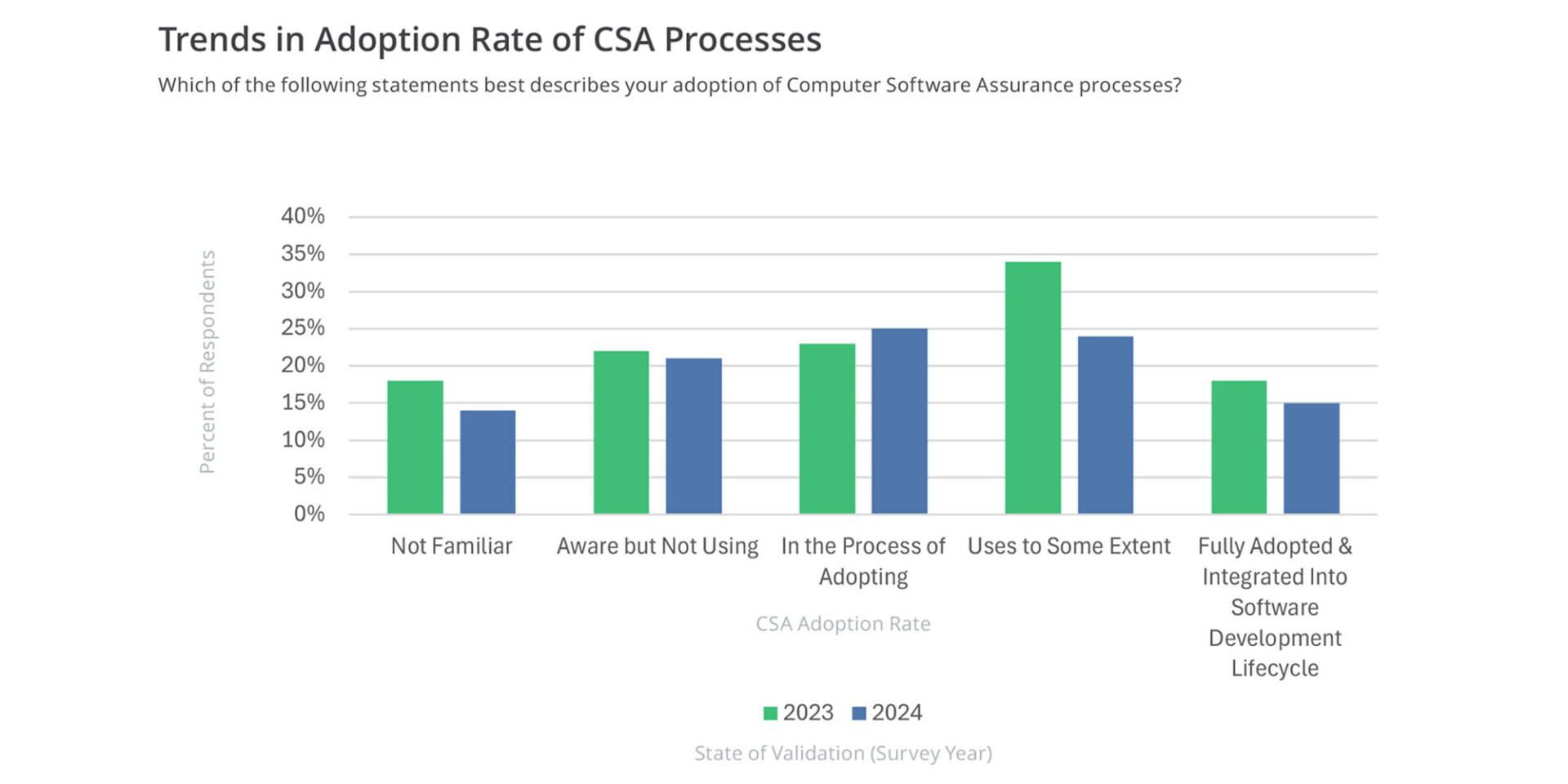
However, there is a decline in those using CSA to some extent (34% to 24%) and in full adoption (18% to 15%). While the percentage of those unfamiliar with CSA has decreased from 18% to 14%, awareness has not significantly translated into usage, with 21% still aware but not using the processes suggesting potential challenges in fully integrating the new approach.
Adopting a CSA approach provides organizations with flexibility and agility in helping ensure that the software is maintained in a validated state. These findings indicate that validation professionals should focus on supporting organizations through the adoption phase and addressing barriers to full integration. Once adopted, professionals should focus on compliance, best practices, and ongoing performance improvement.
Watch our webinar “Utilizing Digitization for Risk-Based CSV” to see how you can leverage risk-based CSV to speed validation.
2. Cloud-Based CSV/CSA Management
In 2024, cloud technology has emerged as a vital tool for managing validation activities. Cloud-based validation platforms provide scalability, flexibility, and secure real-time data access, allowing teams to collaborate seamlessly from different locations. By storing validation data in the cloud, organizations can ensure that all stakeholders have access to the most up-to-date information, which is essential for maintaining compliance and audit readiness.
By implementing your CSV/CSA process using a cloud-based system, companies can also benefit from enhanced data security, automated backups, and simplified disaster recovery. Cloud platforms also enable teams to monitor validation activities remotely, making it easier to oversee complex projects and respond promptly to issues. This trend is accelerating as more life sciences companies recognize the efficiency and cost savings that come with cloud technology.
Kneat leverages specialized cloud automation processes — like infrastructure-as-code, immutable infrastructure, and containerization — to enhance the delivery of our cloud-based digital validation solution, Kneat Gx and provide an easier, faster, and more responsive validation service.
3. Automation for Streamlined CSV/CSA
Automation is also playing an increasingly important role in CSV/CSA, helping organizations streamline processes, reduce human error, and achieve faster results. Automation of test activities is encouraged by regulatory bodies such as the FDA, provided the software supplier is trusted and meets an organization’s quality standards for software systems.
Robust digital validation systems allow companies to execute CSV/CSA tests online, analyze results, and generate compliance documentation more efficiently than manual methods. By automating routine tasks, validation teams can focus on higher-level activities, such as risk assessment and quality control.
Digital validation minimizes human errors through automation in data entry and analysis, enhancing data accuracy and consistency. Automated systems enforce standardized processes, reducing the risk of errors and discrepancies. This ensures reliable data handling, crucial for strategic decision-making and regulatory compliance.
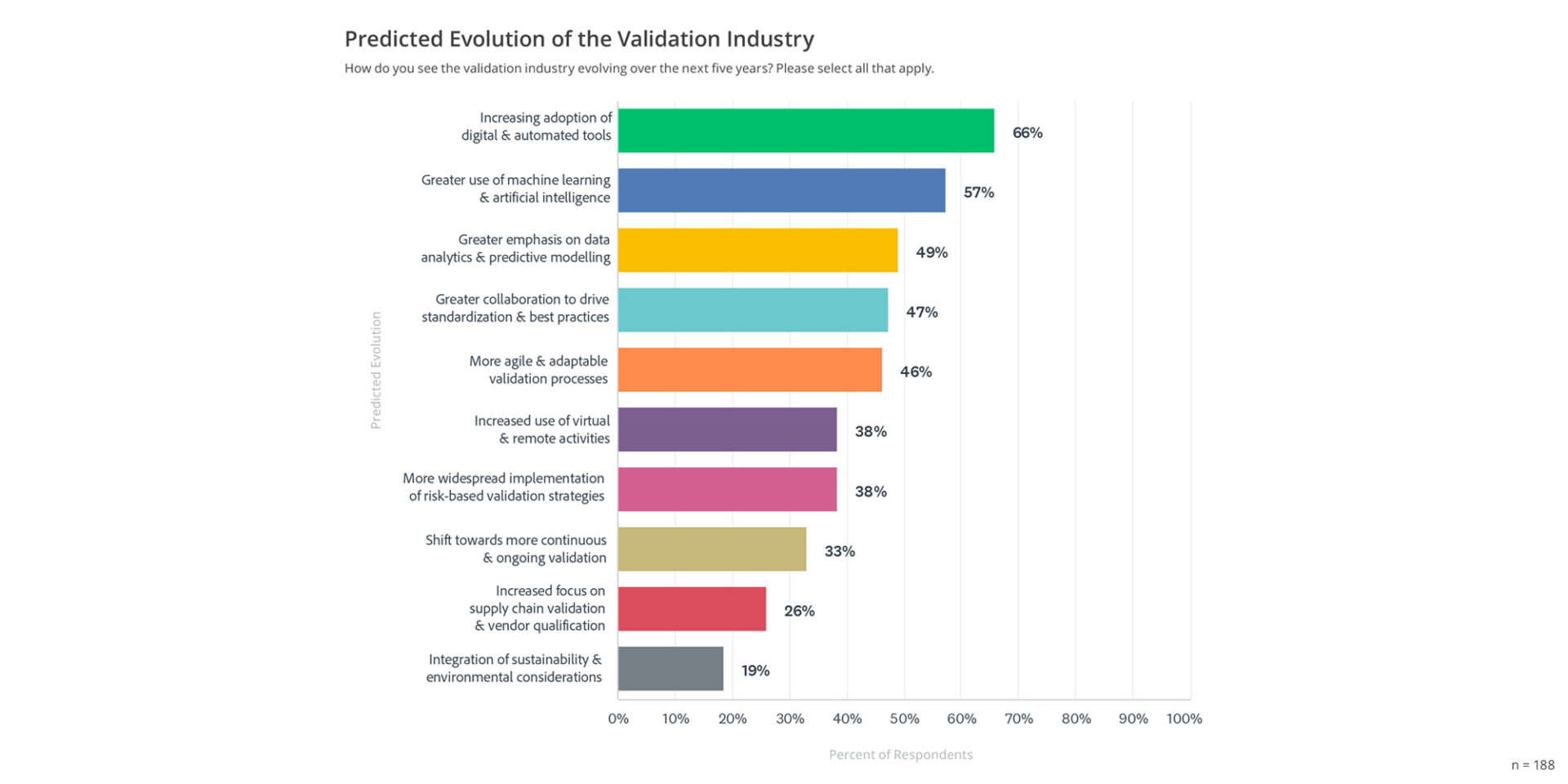
According to the 2024 State of Validation report, a sizable majority of respondents (66%) foresee a rise in the use of digital and automation technologies over the next five years, indicating a shift towards more efficient and streamlined validation processes (see graph below).
Predicted Evolution of the Validation Industry
4. Integration of CSV/CSA With CI/CD Pipelines
As life sciences organizations adopt Continuous Integration and Continuous Delivery (CI/CD) practices, integrating CSV/CSA into these pipelines has become a crucial trend. CI/CD pipelines automate the testing and deployment of software changes, allowing for faster and more frequent releases. By incorporating CI/CD into the CSV/CSA process, companies can validate their systems on an ongoing basis, ensuring that each new release meets regulatory requirements.
Integrating CSV/CSA with CI/CD pipelines also supports agile development, as validation tests are triggered with each change. This reduces the time required for final system validation, as potential issues are identified and addressed early in the development cycle. For organizations looking to accelerate product releases while maintaining compliance, this trend offers a strategic advantage.
5. Using Agile Methodology for Faster Validation Cycles
Agile methodology is gaining traction in the life sciences industry, and CSV/CSA is no exception. Agile emphasizes iterative development, continuous feedback, and rapid adaptability, making it well-suited for dynamic environments like validation. By adopting Agile principles, organizations can break down validation tasks into smaller, manageable sprints, allowing teams to address issues and adjust quickly.
Using an Agile development approach as part of your CSV/CSA process enables more efficient project management and reduces the time required for validation by focusing on short, focused validation cycles. This approach also allows validation teams to collaborate closely with development teams, ensuring that validation activities are aligned with system changes. For organizations looking to reduce validation cycle times and respond more effectively to regulatory updates, Agile offers a flexible and responsive solution.
Appendix D8 of ISPE GAMP5® Second Edition provides a concise guide on how to integrate Agile methodologies within regulated environments. It outlines best practices for combining Agile principles, such as iterative development and continuous feedback, with the requirements of CSV, ensuring both compliance and adaptability.
6. Using Data Visualization Tools for Effective CSV/CSA Analysis
Data visualization tools are becoming indispensable in CSV/CSA, as they allow validation teams to quickly identify trends, spot anomalies, and make data-driven decisions. Visual representations of validation data enable teams to monitor progress, track key performance indicators, and assess the impact of validation efforts in real time. Data visualization also supports regulatory audits, as it provides clear, easy-to-understand insights into validation activities.
By using dashboards and other visualization tools, organizations can simplify complex data sets and gain a comprehensive view of their validation status. This enhances decision-making and improves the ability to communicate validation findings with stakeholders, from regulatory bodies to internal leadership. As the demand for real-time insights grows, data visualization will continue to be a valuable asset in the CSV/CSA toolkit.
Our digital validation platform Kneat Gx delivers at-a-glance insights into every aspect of the validation process across all facilities in real time. You can instantly see if a system is validated, due for review, in review, and more. Business Intelligence dashboards unlock deeper insights including document statuses, user workloads, work processes status updates, across all validation disciplines.
Advanced filter and search functionality complete with preview snippets allows for easy and flexible searching of documents, drawings, files, attachments, and other relevant assets.
Reporting and Paperless Handover in Kneat Gx
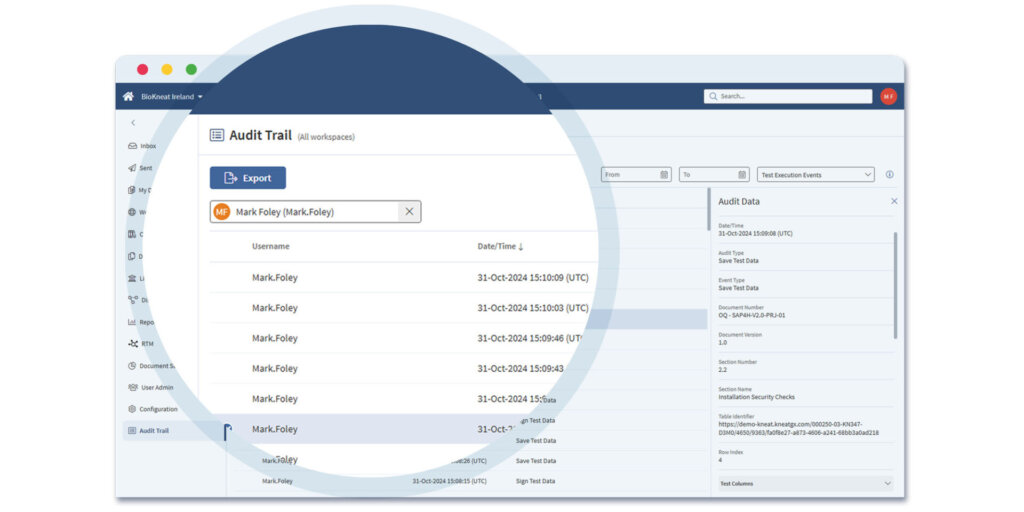
Within Kneat Gx you can run status reports for all projects, systems, and validation deliverables and create a report using filters. Filtered reports are saved and exported to PDF.
Paperless handover makes it easy to present documentation outside of Kneat Gx — perfect for administrative tasks — while including a full audit trail of all activities.
Final Thoughts
CSV/CSA is evolving rapidly, driven by technological advancements and modern methodologies that prioritize efficiency and compliance. From cloud-based validation management and automation of test activities to the use of CI/CD developed processes as part of the CSV/CSA process, these trends are helping organizations streamline validation activities and enhance data integrity. Adopting Agile and risk-based strategies and leveraging data visualization tools are enabling companies to stay ahead.
As organizations incorporate these trends into their CSV/CSA processes, they are better positioned to meet regulatory requirements, reduce operational costs, and enhance overall validation effectiveness.
Our leading validation software Kneat Gx digitalizes the entire CSV/CSA lifecycle, delivering compelling productivity, cycle time, and compliance improvements.
Join us for an exclusive demo and discover how our solution supports the latest trends and makes CSV and CSA faster, easier, and more compliant.
Book your demo today to experience Kneat Gx in action!




