Pharma 4.0 is the term coined by the International Society of Pharmaceutical Engineering (ISPE) that describes the Industry 4.0 shift towards advanced technology including AI and machine learning, cloud computing, and the Internet of Things (IoT). In a pharmaceutical context, these technologies increase production, improve right first time manufacturing, deliver end-to-end visibility, and enhance compliance.
Industry 4.0 refers to the “Fourth Industrial Revolution” which at the highest level can be explained as a widespread adoption of digitalization throughout the manufacturing process. It may be helpful to look back at previous industrial revolutions to understand the scope:
- First Industrial Revolution: Mechanization
- Second Industrial Revolution: Electrification
- Third Industrial Revolution: Automation
- Fourth Industrial Revolution: Digitalization
Digital transformation is another frequent term that has strong relevance in a Pharma 4.0 context, but full adoption is about more than onboarding digital solutions. It’s about having a network of systems working together to provide real-time data collection and adjustments, deeper visibility for stronger business intelligence, and strong data integrity so organizations can move quickly and with confidence.
The Pharma 4.0 Operating Model
As developed by the ISPE, the Pharma 4.0 operating model can be described in four quadrants:
- Resources
- Information Systems
- Organization and Processes
- Culture
Resources
Resources can refer to anything tangible used in the production cycle, from humans to equipment to software that streamlines manufacturing. In a Pharma 4.0 context, resources center around increasing visibility for an organization, including centralizing key business data, increasing traceability of information for improved audit readiness (among other advantages), and enabling predictive maintenance for reduced downtime.
Pharma 4.0 resources will focus on data acquisition and management, bringing more information to decision makers, deeper analysis, and unlocking new opportunities for efficiency. In a validation context, resources play a dual role as they bring more work for validation teams (who need to validate each resource), but also present solutions to help streamline cycle times, enhancing productivity and reducing risk of human error.
Information Systems
As more data becomes available to companies, they’ll need to figure out what to do with it. Data in a silo isn’t usable. Paper-based validation systems showcase this struggle well. For example, if an auditor questions the maintenance of equipment, or requests validation records, that information must be retrieved from under a mountain of boxes bursting with binders of paper records. This is time-consuming, expensive to store, delays production, and increases the risk of a regulatory finding.
Pharma 4.0 seeks to address these constraints, among others, with more centralized data management and integration of systems. This integration and distribution of data brings companies from narrow lens decision making to highly informed strategizing.
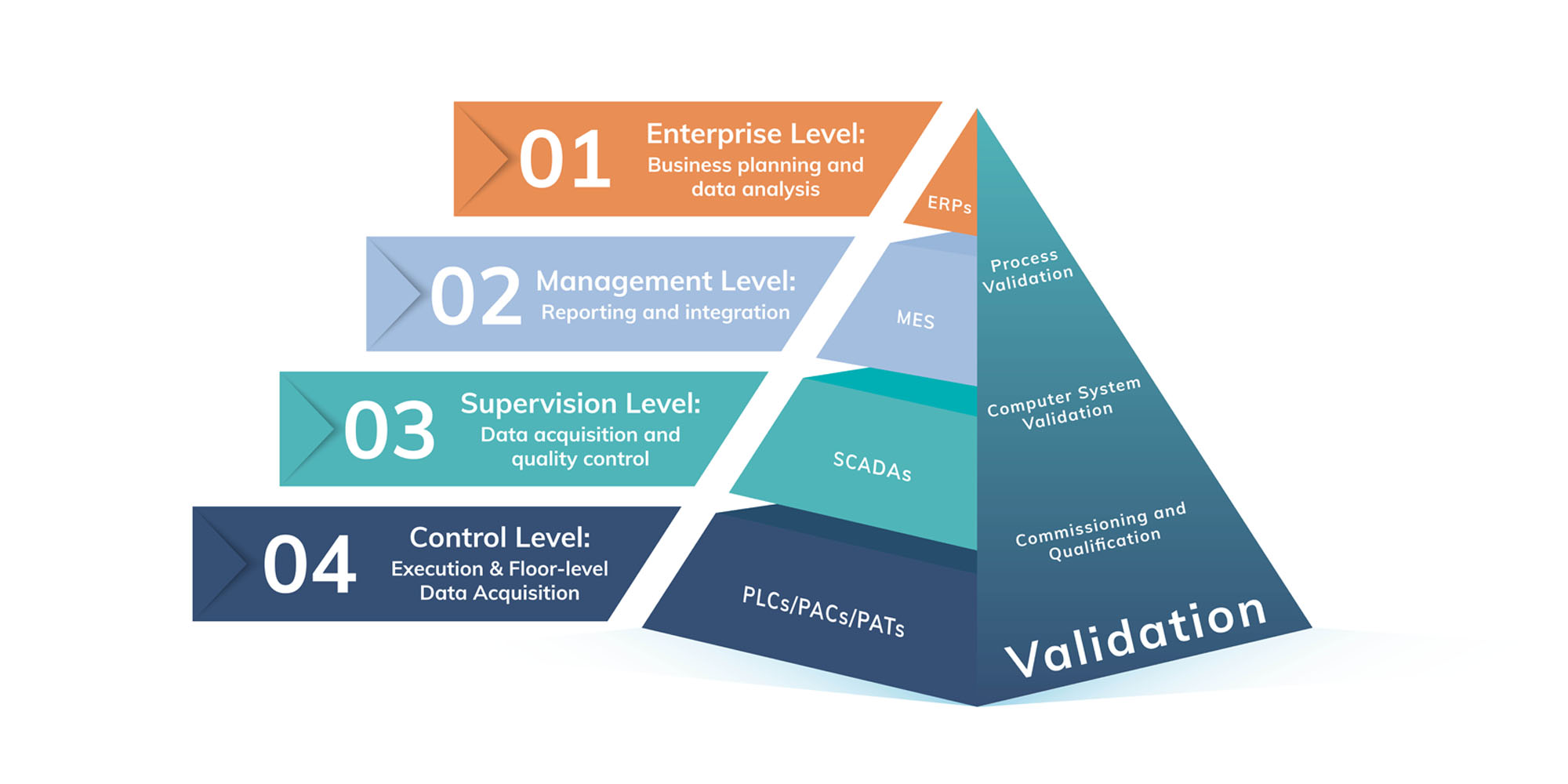
Digital validation platforms like Kneat Gx can integrate with multiple systems, including Manufacturing Execution Systems (MES), Enterprise Resource Planning (ERP) systems, Quality Management Systems (QMS), Document Management Systems (DMS), CAD drawing solutions, and more. When validation lives alongside other areas, especially those owned by validation, quality, or engineering teams, greater efficiency and better visibility is unlocked.
For example, Kneat Gx can identify equipment through Unique IDs, allowing for all related documentation to be accessed at a single click. Validation reports, cleaning records, and maintenance scheduling can inform a schedule for preventative maintenance, reducing operational downtime and driving up productivity.
Another example may be facility walkdowns, where engineers can access engineering drawings on their tablets while on site and make comments or notes and then send them for review. No waiting, no document retrieval. These are just some examples of efficiencies unlocked through Pharma 4.0 information systems.
Organization and Processes
The International Council on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) defines critical quality attributes (CQAs), critical process parameters (CPPs), critical material attributes (CMAs), and key process parameters (KPPs) as essential to safe products and effective designs. They should be monitored by quality and process performance systems to detect deviations or out-of-spec results. This body of information is difficult to collect, manage, and make accessible, but this management is essential to the success of a business.
An Agile organization, including cross-functional teams with interdisciplinary knowledge can leverage an integrated net of GxP-related systems to enhance data management, protect data integrity, and enhance product quality.
Culture
ISPE writes: “Implementing Pharma 4.0 and the holistic control strategy uses the holistic approach to design and execute the business processes and to bring automation and paperless execution to the shop floor.” This involves a culture of alignment between teams in commercial manufacturing, product development, engineering, automation, quality, and IT.
Validation is a task owned by individuals across an organization, with some activities involving any of the above teams. It’s essential that these teams be provided with the most advanced solutions available to perform their duties, record their results, and manage their processes.
Pharma 4.0 Enablers
The ISPE introduced the concept of “enablers” in describing Pharma 4.0 adoption for companies. These describe an organization’s ability to operate within the Pharma 4.0 model. Digital Maturity and Data Integrity by Design.
Digital Maturity
Before we look forward, let’s look back. Pharma 3.0 represented the introduction of mainstream computing. Characterized by digitalization, computerization, connectivity, and stabilizing behavior throughout an organization, Industry 3.0/Pharma 3.0 is a model still used by most companies worldwide.
Using the operating model quadrants we can see how Pharma 4.0 maturity differs:
Resources: Where tools and systems were digitalized, they now provide deep visibility for an organization.
Information Sytems: Where they were computerized, they now provide holistic transparency for stakeholders.
Organization and Processes: Where they connected teams before, they now yield predictive results.
Culture: Where it stabilized performance it now encourages adaptive behavior.
When evaluating any new system, whether it’s for validation, DMS, or beyond, one should consider how it aligns with these Pharma 4.0 principles.
Data Integrity by Design
Data integrity has been a crucial part of the pharmaceutical industry for years, well before the technology we have access to today. It’s a key focus of regulatory agencies around the world, and that scrutiny is increasing. In a Pharma 4.0 model, there’s more data from more systems changing hands more often. Maintaining data integrity is going to be an evolving challenge.
ALCOA+ has been the benchmark for data integrity since its inception and its principles are a core part of digital validation. At a high level, ALCOA+ establishes a minimum standard validation and quality teams must maintain when recording results. It must be clear who did what and when they did it. What they found and how they found it. These records must be time stamped, available easily, and stored long term.
Validation 4.0
Validation 4.0 is a subset of Pharma 4.0, speaking specifically to validation requirements and the needs of validation, quality, and engineering teams responsible for ensuring the efficacy of processes and equipment, and the safety of products and regulatory compliance.
The Pharma 4.0 evolution necessitated a shift in how we execute validation. The pace and complexity of life sciences manufacturing outgrew the capabilities of validation teams using traditional, largely paper-based practices.
We’ve seen encouragement from regulatory bodies to adopt digital solutions to empower validation, notably through GAMP 5 guidance and the FDA’s Computer Software Assurance guidance.
The ISPE describes Validation 4.0 as changing the mindset from siloed validation per systems and equipment, to an integrated validation program.
Digital Transformation in the Pharma Industry
Pharma 4.0 isn’t about a single digital solution, but rather the interconnected web of digital systems all working together to improve production, data accessibility, and quality. Every stage of production has different available solutions, when data is passed from one to the other, the true benefit of Pharma 4.0 is realized.
How Kneat Enables Pharma 4.0
No Pharma 4.0 transformation is complete without a digital validation solution. Kneat Gx is the leading platform that digitalizes your entire validation lifecycle from end to end. Kneat provides a home for all your validation data with enshrined data integrity practices and accuracy. This data can be shared to all your systems involved in production, making Kneat your perfect validation partner for Pharma 4.0.
Learn more about Kneat Gx in our upcoming demo webinar. You’ll see key features and functions that have helped Kneat become the main validation solution for 12 of the top 15 global pharmaceutical companies.







