How to Take Advantage of the Digital Transformation in Pharma
Industry 4.0. Pharma 4.0. Digital transformation. They may be buzzwords, but companies that resist adopting the new technologies and methodologies associated with this revolution will quickly be left behind. In this blog, we’ll discuss how Pharma companies can take advantage of the digital transformation inherent to Industry 4.0.
What Is Industry 4.0?
Industry 4.0 refers to the Fourth Industrial Revolution, characterized by the widescale adoption of digital technologies that improve efficiency, deliver transparency throughout the manufacturing lifecycle, and set the stage for artificial intelligence (AI) and machine learning use in production.
Due to its complicated production considerations and strict regulatory requirements, the pharmaceutical industry, specifically the International Society of Pharmaceutical Engineers (ISPE) developed a Pharma 4.0 operating model to bring Industry 4.0 to the sector.
Digital Transformation in Pharma
There’s no single platform that can bring Pharma 4.0 to life. The digital transformation required involves multi-level integrations between different systems, spread across the production lifecycle. Companies must look for digital solutions that don’t only meet the specific need of the team, site, or product, but also have compatibility capabilities allowing them to pass data from one system to another.
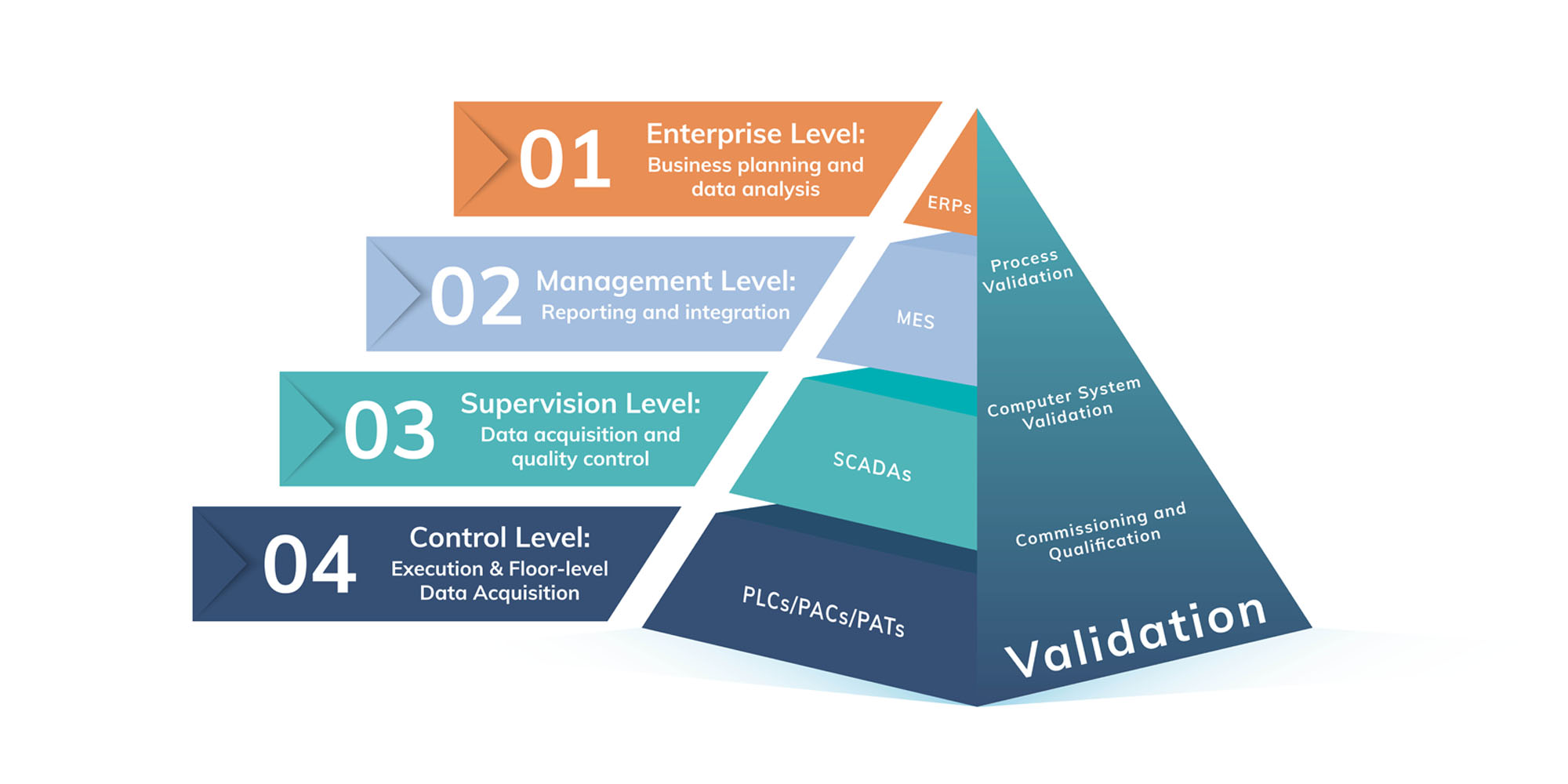
In the Pharma context, this also means that data must stay accurate and attributable, in order to preserve data integrity standards required by regulatory bodies. If we consider the production lifecycle of a pharma company to be divided into four levels, we see the extent of integration required.
- Enterprise Level
- Management Level
- Supervision Level
- Control Level
Enterprise Level
Senior level management and C-Suite executives leverage top-down solutions such as Enterprise Resource Planning (ERP) software. These systems help companies manage critical components of business: finance, HR, equipment and supply chains, procurement, and more. ERPs will change shape to match the needs of the companies leveraging them, but broadly they can be counted on to deliver deep insights through the consolidation of data acquired throughout the organization. At-a-Glance reporting gives planners everything they need, in one place.
They work by integrating modules and applications in a common database. The modules may be specific to a business area, for example HR, or finance. It can be cloud-based, and purchased through subscriptions in a software-as-a-service (SaaS) model, or by license (on premise model).
Management Level
A step down from the high-level view of the Enterprise level for Pharma companies, the Management level tracks production from the ingredient or raw material level until finished production. This is a functional level that provides a buffer for all the data available in production so ERPs (and the executives using them) aren’t overwhelmed by information. The most common system at this level is a Manufacturing Execution System (MES).
Companies leveraging an MES can expect to see improved quality control, increased uptime, improved tracking of production, and paperless reporting. An MES is essential when automating production, which for Pharma companies is critical for continuous manufacturing and batch production.
Data will flow from the site floor via Process Analytical Technology (PAT) equipment and sensors up to the MES. This will help managers track, spot check, and adjust quicker and improve outcomes.
To do this effectively, an MES requires data from the factory floor.
Supervision Level
The software that communicates this data up to an MES is typically a supervisory control and data acquisition (SCADA) system. These gather and process data from sensors and other tools, record events in logs, and help supervisors keep tabs on the equipment making the products.
A key benefit of a SCADA is the rapid detection of deviations or errors. This will alert an operator who can then pause production and use the SCADA to determine where the error is occurring, like a warning light on a car’s dashboard. SCADAs can be simple or complex, depending on the production needs of the organization using them. They’ve developed significantly since the early days of automated manufacturing, and are now built to scale with the incredible data capabilities that underpin Pharma 4.0.
Control Level
The unsung heroes of any digital transformation in the pharma industry is the equipment that monitors, measures, and collects the data that feeds up to the very top of the manufacturing pyramid. Programmable Logic Controllers (PLCs), Programmable Automation Controllers (PACs) along with PAT are these heroes.
These controllers ensure equipment is behaving as intended and thus the product is in a state of control. They are the first to detect when something is amiss, and pass this warning up through the various systems outlined until a remedy is taken.
This unlocks the deep transparency which is the goal of the digital transformation in pharma processes. Enabling more right first time production, which cuts costs and increases production. It ensures quality, removing waste and risk, and empowers decision making to yield yet more gains.
Digital Validation
Another dimension of this extensive integration is digital validation. No digital transformation is complete without it. From the highest level to the base controllers, everything must be tested, validated, and documented. This is unsustainable without a digital solution.
Kneat Gx is the industry leading digital validation solution, providing shortened validation lifecycles for any validation discipline and a validated, compliant system of record for validation data throughout the entire lifecycle.
From the Computer System Validation (CSV) required of MES and SCADA systems to the commissioning and qualification (C&Q) of controllers, Kneat manages all validation needs and integrates with your existing systems.
Learn more in our upcoming webinar to see a demonstration for yourself. Register now.




