In today’s business environment, sustainability has evolved from a trend to a critical necessity, driven by the growing importance of Environmental, Social, and Governance (ESG) criteria. Companies are increasingly held accountable not just for their financial performance but also for their environmental impact, social responsibility, and governance practices.
This shift underscores the need for innovative solutions such as paperless validation for highly regulated industries, which not only streamline operations but also contribute to sustainability goals.
This article explores the concept of paperless validation, its benefits, its role in promoting sustainability through green validation practices, and future trends.
What Is Paperless Validation?
Paperless validation, also known as digital validation, is the process of verifying and documenting the compliance of systems, processes, and products without using paper. Unlike traditional validation methods that rely heavily on printed documents and manual entries, paperless validation leverages digital tools and software to streamline the validation process.
Traditional validation often involves stacks of paper documents, manual data entry, and significant human intervention, leading to inefficiencies and a higher risk of errors. In contrast, paperless validation uses electronic records, automated workflows, and digital signatures to ensure that all validation activities are accurate, efficient, and compliant with regulatory standards.
The Role of Paperless Validation Software
Paperless validation software is at the heart of this digital transformation. These software solutions are designed to automate and manage validation processes electronically. They support validation activities, allowing for faster execution and contributing significantly to reducing environmental impact. A robust paperless validation solution, like Kneat Gx , offers many key functionalities, including:
- Real-Time Analytics: Provides instant analysis and reporting capabilities, enabling quicker decision-making and issue resolution.
- Comprehensive Documentation: Maintains all validation records electronically, ensuring easy access, retrieval, and audit readiness.
- Digital Signatures: Ensures the integrity and authenticity of validation documents with secure, 21 CFR Part 11 compliant, electronic signatures.
Green Validation
Implementing a paperless validation system offers benefits that extend beyond operational efficiency. Green validation refers to the practice of adopting environmentally-friendly validation processes. By leveraging paperless validation, companies can achieve green validation, aligning their operations with sustainable practices.
Benefits
- Resource Conservation: By reducing paper usage, companies conserve natural resources, such as trees and water, used in paper production.
- Waste Reduction: Electronic documentation reduces the amount of waste generated by validation activities, contributing to a cleaner environment.
- Lower Carbon Footprint: Digital processes reduce the carbon footprint associated with printing, storing, and transporting paper documents. This contributes to a cleaner, more sustainable environment.
- Practices: Implementing digital tools and processes supports broader sustainability initiatives, helping companies achieve their ESG
See how CSV Life Science, a Kneat strategic partner, uses Kneat Gx to achieve a more sustainable approach , eliminating paper usage.
Kneat Gx is better for our well-being, better for the planet, and more cost-effective.
Customer Validation Lead, Top 20 Global Biopharmaceutical Company
Digital validation will not only help us from the point of view of paper reduction, but more importantly…this will result in a significant reduction in emissions from the less travel that will be required.”
Pablo Fernando Muñoz Pierattini, Computer System Validation Manager, CSV Life Science
Reduction in Validation Effort
Kneat Gx customers have reported a significant reduction in validation efforts, with validation work hours decreasing by up to 50% in some cases.
Read our case study, Top 20 Global Biopharmaceutical Company Boosts Efficiency, Data Integrity, and Auditability, to discover how Kneat Gx delivered significant efficiency gains, enhanced protocol management, and an extremely positive audit experience with inspectors and users.
Cost Savings
- Lower Storage Costs: Electronic documents eliminate the need for physical storage space, reducing costs associated with filing cabinets, storage rooms, and archiving.
- Reduced Printing Costs: Eliminating paper reduces expenses related to printing, including paper, ink, and maintenance of printing equipment.
- Streamlined Audits: Digital records make it easier to retrieve and review validation documents during audits, saving time and reducing audit-related costs.
In The Digital Validation Handbook: Your Guide to Faster, More Accurate Validation, you will find out everything you need to know about how to streamline validation, leave paper-based processes behind, mitigate noncompliance risks, and drive more value for your company.
Future Trends in Paperless Validation and Sustainability
As technology continues to evolve, several emerging trends are shaping the future of paperless validation and sustainability:
Increased Adoption of AI and Machine Learning
Artificial intelligence (AI) and machine learning are revolutionizing paperless validation by enabling predictive analytics and automating complex validation tasks. These technologies enhance accuracy and efficiency, making validation processes more robust and reliable.
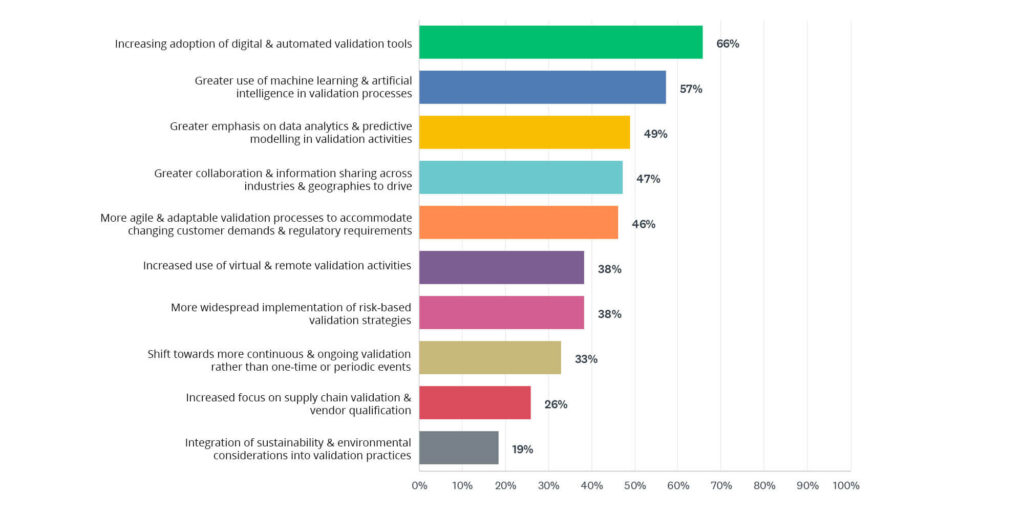
2024 State of Validation survey data reveals which new technologies professionals believe will be used in the validation industry. Artificial Intelligence and Machine Learning (70%) is the most anticipated technology, indicating a strong belief in the transformative potential of AI and machine learning to enhance validation processes through automation, predictive analytics, and intelligent decision-making.
Pharma 4.0 Adoption
The pharmaceutical industry is embracing Pharma 4.0, which integrates digital technologies to create smart, connected systems. Paperless validation not only complements your Pharma 4.0 initiatives but also helps you meet your requirements over 40% faster, offering the necessary interconnectivity and flexibility to thrive in the Pharma 4.0 landscape.
According to the 2024 State of Validation study, digital validation system users are further along in the adoption of Industry/Pharma 4.0 technologies, with more advanced implementation and full integration:
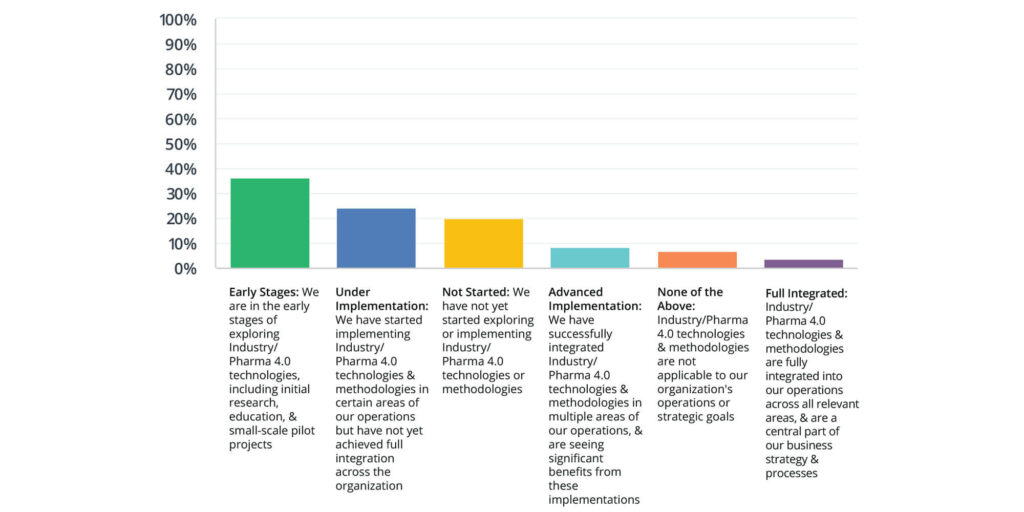
Non-digital users are predominantly in the early planning stages or have not started, highlighting a slower transition towards adopting these advanced technologies.
This trend towards Pharma 4.0 is enhancing paperless validation by enabling real-time data sharing, predictive maintenance, and advanced analytics, thereby improving product quality and compliance.
Final Thoughts
Paperless validation is a transformative approach that not only enhances operational efficiency but also supports sustainability and green validation practices. By transitioning to paperless systems, companies can reduce their environmental impact, achieve cost savings, and improve compliance.
As emerging technologies continue to shape the future of validation, businesses that adopt these innovations will be well-positioned to thrive in an increasingly competitive and environmentally conscious marketplace.