The FDA issued its final Computer Software Assurance for Production and Quality System Software Guidance on September 24, 2025, replacing the 2022 draft and cementing a modern, risk-based approach for assuring non-product software that supports device production and the quality system.
We break down nine practical shifts from draft to final and show how Kneat Gx operationalizes each one with digital evidence, traceability, and inspection-ready records.
1. Cloud is explicitly “in play” via worked SaaS examples
The final guidance includes an Appendix A case for a SaaS Product Lifecycle Management (PLM) system and describes establishing service agreements with the SaaS vendor as part of assurance, making cloud deployments unmistakably in scope.
- Why it matters: If your QMS or production relies on SaaS, you can apply CSA’s risk framework, configuration verification, and exploratory UAT to right-size assurance.
- How Kneat Gx helps: Kneat runs on secure cloud infrastructure with audit trails, configurable templates, and remote review/approval-supporting global, multi-site assurance for SaaS-connected processes.
2. Stronger guidance on the record you need (goodbye screenshot sprawl)
The final guidance spells out a prescriptive “appropriate record” recipe: intended use, risk-based analysis, objectives tested, testing performed, issues found, conclusion, and who/when.
- Why it matters: Teams can standardize lean, digital records that are inspector-friendly without over-documenting low-risk functionality.
- How Kneat Gx helps: Kneat templates capture all required record elements with ALCOA++ integrity, auto-timestamps, and auditable approvals-reducing manual evidence and rework.
3. Scripted vs. unscripted vs. hybrid testing tied directly to process risk
The examples explicitly endorse exploratory (unscripted) testing where risk is not high, showing concise acceptance narratives and deviations handled through simple rules.
- Why it matters: High-risk features still justify scripted rigor, but much of your ‘not high-risk’ software can be assured faster with exploratory or scenario-based testing.
- How Kneat Gx helps: Kneat captures exploratory outcomes, deviations, and approvals inline with full audit trails, so lightweight assurance never compromises traceability.
4. Clearer Part 11 alignment without myth or mystery
FDA directs manufacturers to the Part 11 Scope & Application Guidance and explains when electronic records and signatures apply to production/QMS software-separating predicate-rule evidence from routine logs.
- Why it matters: You can focus Part 11 controls on the electronic records that actually constitute required evidence supporting a validated state.
- How Kneat Gx helps: Kneat provides Part 11-ready e-records, e-signatures, and exportable audit trails mapped to predicate-rule evidence, simplifying inspection narratives.
5. A crisper, risk-based message baked into structure
The final guidance organizes CSA as a risk framework: identify intended use, determine risk, select activities, and establish the record-making “no more than necessary” the operational default.
- Why it matters: This structure lets you standardize how teams justify why this testing, not that, improving consistency across sites and suppliers.
- How Kneat Gx helps: Real-time RTM, risk fields, and standardized templates keep rationale, tests, and evidence connected-so justifications are consistent and visible.
6. Examples that look like what you actually do
From spreadsheets used to monitor nonconformances to SaaS PLM and LMS, Appendix A gives short, reality-based assurance write-ups you can emulate immediately.
- Why it matters: Models make adoption faster. Your teams can mirror FDA-style structures without reinventing content or debates.
- How Kneat Gx helps: Kneat’s template packs encode these patterns, so authors capture intended use, risk rationale, activities, deviations, and conclusions consistently.
7. Digital evidence is first-class, not an afterthought
The record guidance and examples emphasize objective, attributable, time-stamped information rather than piles of screenshots.
- Why it matters: Systems that generate native digital evidence speed reviews and reduce documentation debt while staying inspection ready.
- How Kneat Gx helps: Kneat’s ALCOA++ implementation-time stamps, immutable audit trails, and direct data entry-ensures evidence is created and controlled at the source.
8. Practical clarity on what’s in CSA scope
The guidance targets software used as part of production or the quality system, distinguishing these uses from general business tools and showing how to justify risk where patient safety is not foreseeably impacted.
- Why it matters: Scope clarity prevents over-validating lower risk business tools while ensuring QMS/production software stays in a validated state.
- How Kneat Gx helps: Kneat’s process catalogs and configurable document types let you classify use cases, apply proportionate activities, and maintain state of control across changes.
9. Inspections will expect consistency, not maximalism
The risk framework and record elements signal inspectors will look for justified, consistent assurance over exhaustive artifacts for low-risk functions.
- Why it matters: Harmonizing how teams document CSA reduces finding risk and review time across global programs.
- How Kneat Gx helps: Kneat’s RTM, standardized workflows, and remote review compress cycle times while improving audit readiness.
Adoption momentum is already here
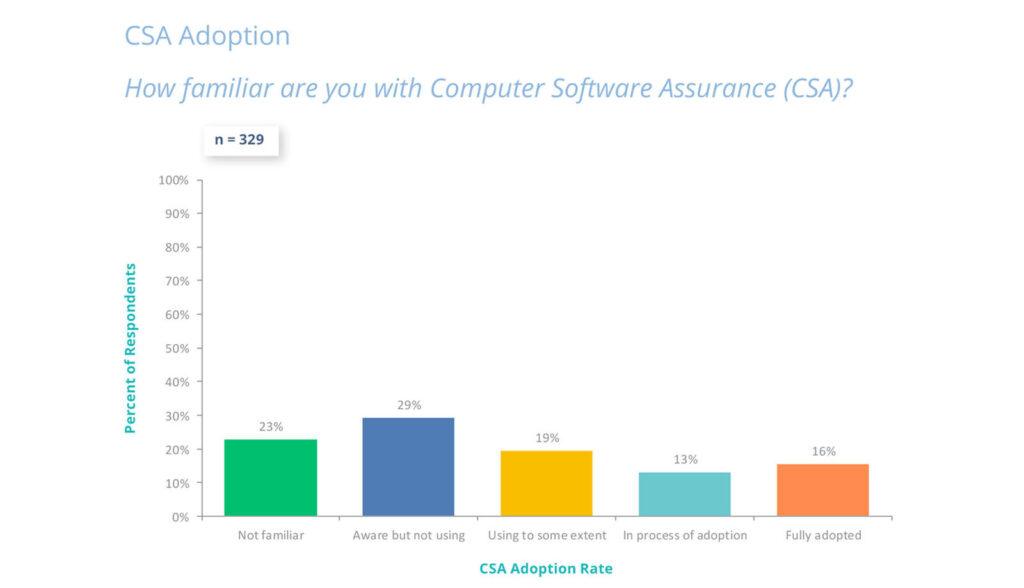
The 2025 State of Validation Industry survey shows nearly half of respondents are adopting or using CSA processes, with digital validation prioritized for audit readiness and data integrity.
- Why it matters: With the final guidance now published, organizations can finish replacing CSV habits with CSA practices that reclaim time and budget.
- How Kneat helps: Kneat customers report reductions in validation labor hours and process steps, enabled by centralized digital evidence and automation.
From paper to proof: build FDA’s “appropriate record” in Kneat
Map each FDA element: intended use, risk-based analysis, objectives, testing performed, issues, conclusion, and who/when-to fields in a Kneat template, and you’ll generate a compact, Part 11-ready record by design.
Kneat auto-captures ALCOA++ attributes with time stamps, user identity, audit trail, and e-signatures, eliminating backdating risk and making true copies and revisions transparent. With real-time RTM, reviewers can jump from a requirement to its test and evidence instantly, speeding approvals and inspections.
Right-sizing testing with confidence
Reserve scripted testing for higher-risk functions and use exploratory or other unscripted methods when risk is not high — documenting the same core record elements FDA outlines (intended use, risk-based analysis, summary of testing performed, issues, conclusion, and who/when).
Kneat Gx lets you implement a single, reusable template that captures FDA’s record elements consistently across modalities, supporting standardization without changing FDA’s intent.
Why Kneat Gx now
Kneat Gx provides secure access control, Part 11-ready e-records and signatures, fully exportable audit trails, and configurable templates that mirror FDA’s record recipe.
Teams see faster cycles through automation, real-time test execution, and remote review/approval, delivering measurable time savings. The platform’s ALCOA++ alignment, RTM, and centralized evidence make least burdensome the default, not the exception.
If you have not shifted to CSA, you are doing yourself a massive disservice. In 2022, we started on our journey to take advantage of the draft guidance across our entire validation program, FUE, Utilities, CSV, Equipment. Kneat has been terrific. We developed our templates in Kneat to drive critical thinking. I am extremely pleased.
Director, Computer System Validation, Industry Leading CDMO
The bottom line
With the final CSA guidance in force, you can standardize lean, risk-based assurance for production and QMS software. Kneat Gx is the fastest way to turn that guidance into day-to-day, inspection-ready practice.






