Validation in the consumer health industry has become increasingly complex. Positioned at the crossroads of healthcare and consumer goods, the sector must meet pharma-grade regulatory expectations while maintaining the speed and flexibility of consumer manufacturing.
Rising pressure on validation teams
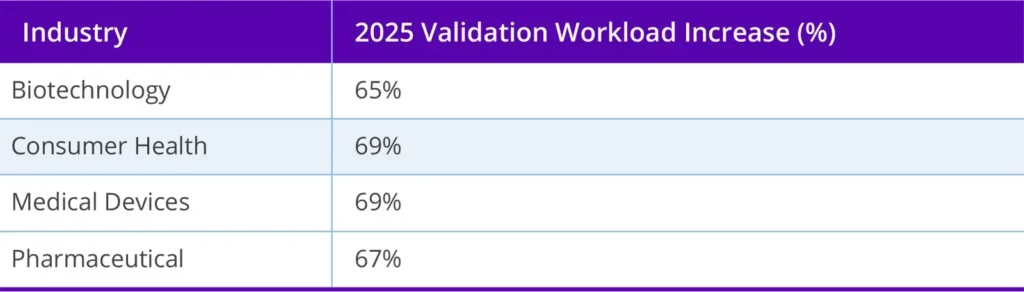
According to the 2025 State of Validation study, 69% of consumer health professionals reported an increase in validation workload this year, a trend consistent with the broader life sciences landscape (see table below). These teams often operate with limited resources — 46% manage with six or fewer validation professionals — yet face mounting global compliance demands.
Common challenges include:
- Regulatory complexity: Organizations must comply with FDA and EMA frameworks, consumer product standards, and regional health regulations simultaneously.
- Rapid product turnover: Seasonal reformulations, packaging updates, and new product variants all trigger additional rounds of validation.
- Distributed operations: Global supply chains make it difficult to harmonize validation processes and ensure data consistency across sites.
- Audit pressure: Regulators expect continuous inspection readiness with robust data integrity.
- Resource constraints: Lean teams must do more with less, relying on manual or hybrid systems that slow them down.
From challenge to opportunity: the shift toward digital validation
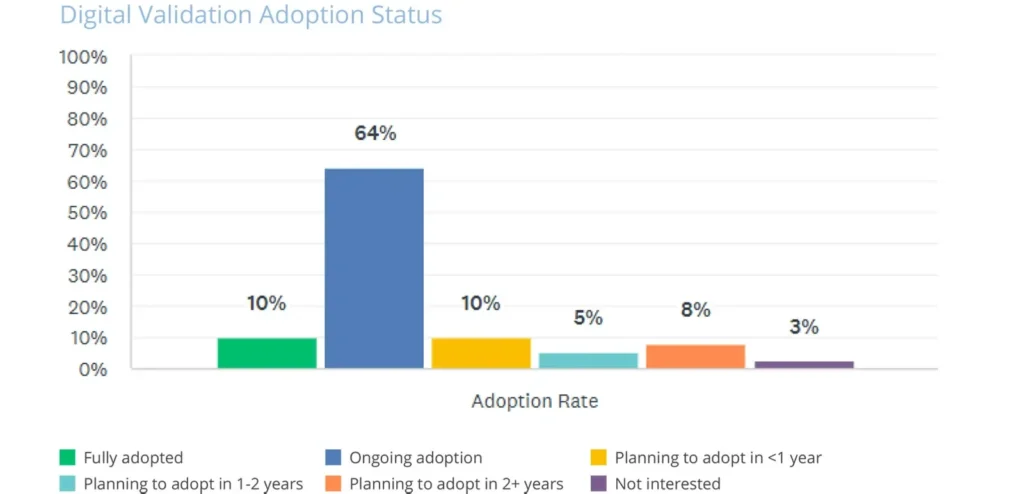
Despite these pressures, the consumer health industry is embracing digital transformation. The State of Validation data shows that three in four consumer health organizations have either adopted or are in the process of adopting digital validation systems, with only 3% reporting no interest (see graph below).
The benefits are clear:
- Data integrity ranked as the number one advantage of digital validation.
- Continuous audit readiness and global standardization followed closely behind.
- Nearly two-thirds of early adopters say digital validation met or exceeded ROI expectations.
Digital systems such as Kneat Gx replace paper-based workflows with secure, compliant, and fully traceable digital processes. That means fewer manual errors, faster approvals, and simplified audits — critical in a sector where speed and safety must coexist.
A look at the OTC and generics segment
Within Consumer health, OTC and generics manufacturers face additional pressure to meet tight timelines, strict regulations, and high-volume output. Traditional paper-based validation methods are often too slow and error-prone to keep up.
Kneat’s eBook, Digitalizing Validation for OTC & Generics Manufacturing, highlights how digital solutions solve these pain points by enabling:
- Online test execution and real-time deviation management
- Automated traceability matrices for continuous compliance
- Electronic logbooks that capture every action securely and accurately
- Centralized audit-ready records that eliminate redundant effort
Customers using Kneat Gx have achieved:
- 65% reduction in validation labor hours
- 47% of process steps removed
- 17% faster speed to market
These gains translate directly into more compliant, more agile operations — and faster product launches without sacrificing safety or quality.
Building the future of consumer health validation
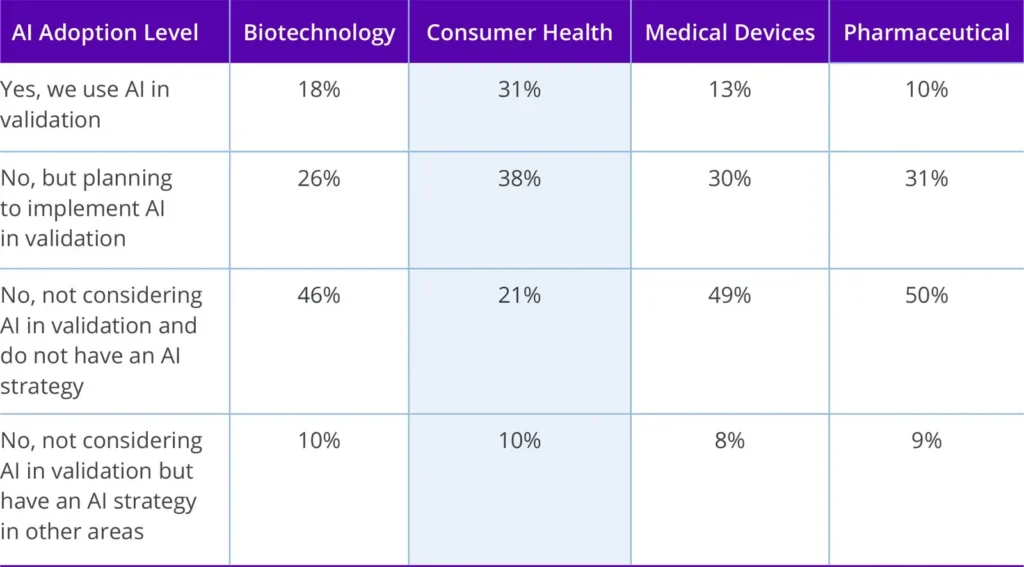
As digital transformation accelerates, AI and Computer Software Assurance (CSA) are emerging as the next frontier. Nearly one-third of Consumer health organizations are already using AI in validation, the highest rate across life sciences sector (see table below). The top use cases include data analysis, protocol optimization, and predictive maintenance.
While regulatory frameworks continue to evolve, the direction is clear: Consumer health companies that invest now in digital-first validation strategies will be better positioned to scale, remain compliant, and innovate faster.
The Kneat advantage
Kneat Gx helps Consumer health organizations transform validation from a compliance requirement into a strategic advantage. With guided workflows, automated traceability, and secure cloud storage, teams can standardize globally while maintaining flexibility at the site level.
Whether you’re managing OTC manufacturing, dietary supplements, or global consumer brands, Kneat Gx delivers the solutions to simplify compliance, strengthen data integrity, and accelerate time to market.
See Kneat Gx in action — book a personalized demo and discover how digital validation can power the next generation of Consumer health innovation.






