Building off the success of the inaugural VALIDATE conference in Boston last November, Kneat held its first annual European conference VALIDATE EU, in Dublin on April 18-19, 2023. Purpose-built for validation, quality, and compliance professionals, the event brought over 150 experts and digital validation leaders together in one place to share their knowledge and first-hand experience.
We had 16 expert-led sessions, some delicious food, and fun networking events in Dublin at the Marker Hotel and the Guinness Storehouse, with insightful panels and workshops led by key leaders and innovators from across the life sciences industry. A huge thank you to all who traveled to Dublin to participate! Watch the video now to see a recap of VALIDATE EU 2023.
During VALIDATE EU, we heard from some of the world’s biggest life sciences companies and more than 30 valued partners — including AbbVie, Amgen, Fedegari, Gilead Sciences, GSK, Lonza, and Novartis. The conference theme of ‘Data Driven Quality’ attracted tech-forward speakers who covered topics including optimizing manufacturing performance and quality using data, enabling smarter quality assurance and validation through data governance, and achieving standardization and organizational agility through digital validation.
We also heard from Kneat experts who talked about digital validation best practices, digitizing computer system validation (CSV) to enable efficient computer software assurance (CSA), and how to shift your focus from documentation to data. For existing Kneat customers, sessions on Kneat’s product roadmap provided a chance to sit down and explore how to further extend and customize Kneat Gx to meet their business needs.
We came away from VALIDATE EU with more ideas than ever to expand and transform the digital validation playbook. Here are our five key takeaways from the conference. We hope they serve as a reminder of what’s possible when validation, quality, and compliance professionals and highly regulated companies come together with the right technology, tools, and services.
1. Knowledge Sharing Delivers Impactful Outcomes

To launch VALIDATE EU, Kneat CEO Eddie Ryan’s opening remarks reflected on how sharing knowledge of digitizing validation — whether you are embarking on, or accelerating, your digital validation journey —can enable impactful outcomes for the Life Sciences industry by:
- Bringing patient therapies to market faster to high quality and compliance standard
- Lowering CQV lifecycle costs and schedules
- Streamlining and harmonizing best practice across organizations
- Delivering real-time visibility and collaboration across your organization
- Including all stakeholders, partners, and vendors
2. Harnessing the Power of AI Requires Data Driven Quality

Artificial intelligence is probably the most disruptive technological development in human history, and it is ubiquitous; from the face recognition on your smartphone to advanced medical research, from payment fraud detection to industrial robots.
In addition, other emerging technologies such as the internet of things, blockchain, and virtual reality complement and interact with artificial intelligence to create even more immersive, complex, and disruptive products and services.
But what is AI really, what is it not, and how can the life science industry benefit from the advantages whilst abiding by the requirements for quality control, traceability, and compliance? Our keynote speaker, Elin Hauge, renowned futurist and AI expert discussed the challenges and opportunities of harnessing the power of AI in the Life Sciences industry.
The key takeaway? Data quality is essential to avoid the confusion matrix, when building your AI tool kit.
“Elin made me rethink my opinion of AI from negative to more positive. I would still be cautious of each application where AI is used but I think it is very valuable in the right space.”
– VALIDATE EU attendee, CQV Validation Engineer
3. Change Management Is Enhanced by Digitization

David O’Connor, C&Q Digital Transformation Manager at No deviation, moderated a robust panel discussion which featured Denis Kelleher, Validation Engineering at Gilead Sciences, Patrick Scheidegger, Global Head of CQV for SGIE at Lonza, and Craig Wilson, Senior Validation Manager at Amgen. Our panelists discussed how validation leaders can best manage change arising from ever-increasing use of digital technologies and help resolve the growing human resources challenges facing validation teams. They also considered what training digital validation professionals need to keep up with change in the validation industry.
Kneat’s Project Management Office Manager, Paul Fahy, explains how his team works with our customers: “It is imperative to have key decision makers part of the customer project team. Too often projects get slowed down by not having the correct people empowered to make the decisions required; such as process harmonization, globalization, site adoption etc. For successful adoption, an engaged Project Sponsor has proven to be of real importance to champion the project for the business, ensure the team is adequately resources and remove obstacles as needed.
“Training the client project team to Power User 1 level ahead of project-kick off is an important milestone to achieve. This training provides an understanding of decisions needed and provides context to discussions regarding the move from a paper-based system to a digitized solution.
“Once implemented, having the correct infrastructure in place to support the introduction of Kneat has proven to be of value. This included having sufficient training provided throughout the organization and adequately resourcing the end user on site. Not providing sufficient support can become a bottle neck as adoption outpaces support.”
As digital validation cements itself as the new normal, validation professionals face the inevitable challenge of guiding their organization through change to a digital future.
4. Digitizing CSV Enables Efficient CSA

The FDA recently released its Draft Guidance for Computer Software Assurance (CSA) for Production and Quality System Software. Kneat’s CSV specialist, Darren Geaney, walked VALIDATE EU attendees through the changes and what they mean for validating computer systems in his session, ‘Digitizing Computer System Validation to Enable Efficient Computer Software Assurance’.
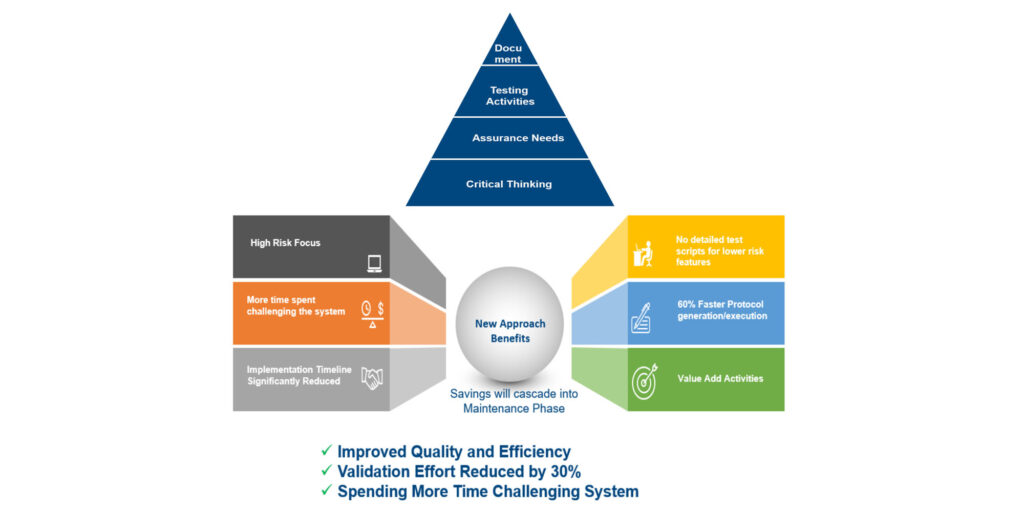
Darren outlined the benefits of CSA guidance, which include the ability to:
- Leverage the vendor — leverage audits and testing by the vendor to further scale validation effort
- Perform risk-based evaluation — scale validation activities based on risk
- Streamline testing — all functionality is tested but only high-risk functionality requires traditional scripted testing
- Utilize unscripted and ad-hoc techniques — for low/medium risk
Benefits of CSA Guidance
He also shared some of the benefits of digital validation for CSA, which should automate the validation lifecycle processes and lead to elimination of inefficiencies while ensuring consistency and compliance to SOPs and regulations. Further advantages include:
- Cycle time reductions in review and executions
- Data integration and safe and secure storage of generated data
- Reduction in time taken to generate electronic traceability matrix
- Integration of validation and change management processes
- Cost reductions for printing and storage of paper records
- Increased Quality
- Scalability of the system to add new software systems, sites, etc.
- Instant access to validation artefacts in the event of audit
Watch Darren’s on-demand webinar to learn more about achieving CSA with digital validation.
5. Project Management Know-How Is Key to Building a Successful Business Case for Digital Validation
Critical to the success of implementing a digital validation solution is project management know-how and the ability to communicate business and financial benefits to gain executive buy-in.
Graham Cameron, Engineering Lead, CQS at GSK, Donncadh Nagle, Assistant Lecturer, School of Chemical & BioPharmaceutical Sciences, Technological University Dublin, Simone Riva, Innovation Manager at Fedegari, and Rick Mineo, Director Strategic Partnerships & Kneat Academy at Kneat, outlined what validation professionals need to know about their organization in order to create an effective business case, some of the key pitfalls and learnings from their own experiences with building business cases for validation software, and digital validation project management best practices, in their panel discussion on ‘Creating a Business Case for Your Validation Software Project’.
According to our panelists, effective business cases should:
- Identify a known business problem or objective
- Propose a solution to that problem
- Outline a project to provide that solution
- Forecast a return on investment or net present value of the proposed project; and
- Communicate other non-revenue related benefits
You can learn more about how to build a successful business case for your project in our Digital Validation Budgeting Guide.
Thank You to All Our VALIDATE EU Partners
Thank you to all our speakers, partners, and attendees for making VALIDATE EU 2023 a success. And a huge thank you to our sponsors, CAI, No deviation, VEQTOR, PharmEng Technology, and PiQuP for your support.
“Thank you for hosting a great conference and for providing a forum for us all to discuss Kneat in person with our peers. It was fantastic to meet the Kneat team and very reassuring to know that progress will continue to meet industry requirements.”
– VALIDATE EU attendee, Senior Process Scientist




