Use case
Commissioning and qualification
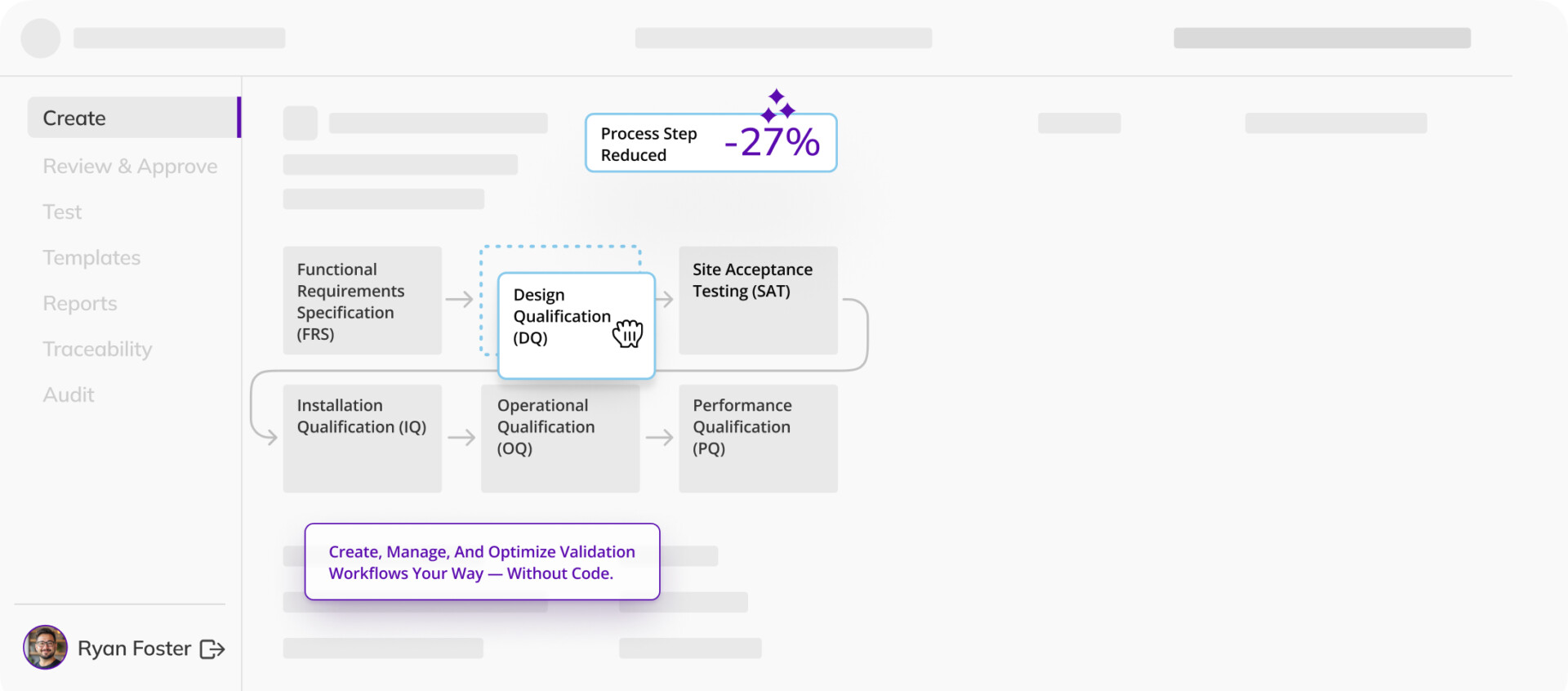
Ensure facilities, systems, and equipment are designed, installed, tested, and operated per requirements — as efficiently as possible.

Trusted by industry to improve efficiency and reduce compliance risk.

Flexible & Compliant

We were able to demonstrate over 50% cycle time reduction…and process simplification from 15 steps to 8 … because of Kneat we minimized the number of systems we used from 5 to 2.
- Global Executive Director, MSD
Book a demoCustomer Success
Use Cases
A single platform for commissioning and qualification, equipment validation, computer system validation, and more.
Use case
Ensure facilities, systems, and equipment are designed, installed, tested, and operated per requirements — as efficiently as possible.

Use case
Ensure computer systems are validated, tested, and maintained in compliance with requirements. Guaranteeing efficiency and effectiveness throughout their lifecycle.

Use case
Ensure equipment is validated, tested, documented, and maintained to meet operational and regulatory requirements. Assuring consistent and efficient performance.

Use case
Ensure analytical instruments are qualified, calibrated, and maintained to provide accurate, reliable, and traceable results.

Use case
Maintain a state of continuous compliance and documentation accuracy to ensure preparedness for regulatory inspections and all forms of audits.

Engineering teams choose Kneat to streamline commissioning and qualification, ensure equipment integrity,
and accelerate
delivery timelines.

Quality assurance teams rely on Kneat to ensure data integrity, protect regulatory compliance, and enable audit readiness.

IT teams implement Kneat to replace multiple point solutions and integrate with their digital ecosystem.

Quality control teams choose Kneat to seamlessly collaborate and contribute to critical validation activities.

Customer Ratings
G2.com
Overall rating by customers via independent industry analyst firm, g2.com.
CSAT Score
97% of our customers rate our customer support as either ‘Very Good’ or ‘Excellent’.
PLAN TO RENEW
Customer feedback via industry analyst SoftwareReviews.com.
Reduce cycle time in every step of your validation lifecycle, for any process. From protocol creation to review, approval, test execution, and more.
Enjoy peace of mind with complete traceability, instant document retrieval, and audit ‘war-rooms.’ Ensuring confidence and readiness for inspection anytime.
Ensure unparalleled data integrity with secure, time-stamped audit trails, role-based access controls, and automated traceability. Enhancing compliance and trust in your validation processes.
Kneat is easy to use and learn, enabling your organization to make a lasting change to digital validation faster, and more easily.
Digitalize validation, your way with the validation platform trusted by the world’s leading life sciences companies.
Talk to an expert