The 2024 State of Validation Report provides predictions about the future of validation in life sciences and other highly regulated industries. Section Five of the report focuses on emerging technologies and methodologies and discusses the anticipated evolution of the industry over the next five years, along with upcoming challenges.
Here are key takeaways from the report regarding these emerging trends, challenges, and opportunities.
1. Adopting Industry/Pharma 4.0
Industry/Pharma 4.0 is a digital transformation approach that integrates advanced technologies like digital validation systems, artificial intelligence (AI), the Internet of Things (IoT), robotics, and big data into manufacturing and validation processes. This approach aims to enable organizations to operate more efficiently and adapt quickly to regulatory and market changes.
The 2024 State of Validation Report reveals that 36% of organizations are in the early stages of Industry/Pharma 4.0 adoption, with 24% actively implementing digital tools, 9% in an advanced stage of implementation, and 4% fully integrated (see graph below).
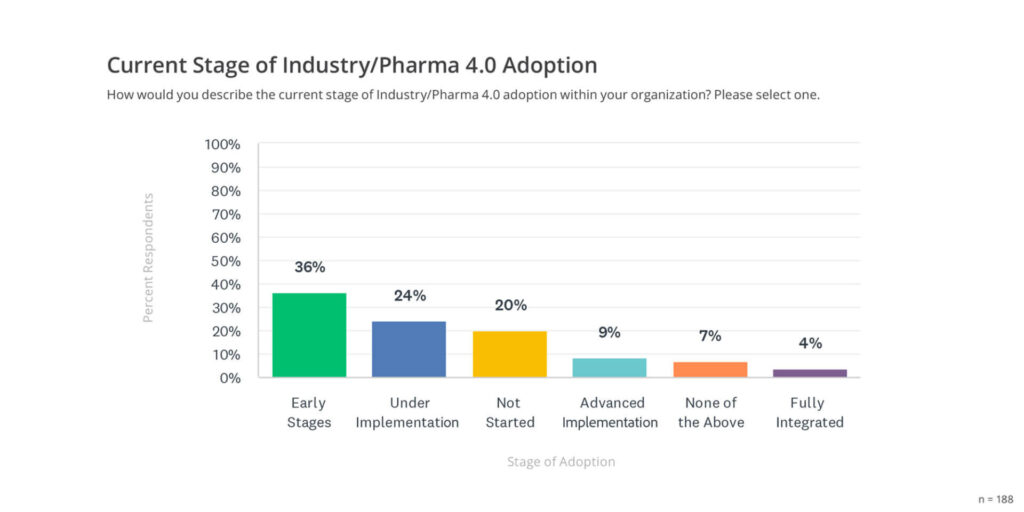
This digital transformation not only drives efficiency but also positions companies to proactively address emerging challenges, ensuring a robust, future-ready validation framework.
2. Increasing Use of Digital and Automated Tools
A sizeable trend is the ongoing adoption of digital and automated validation tools, with 66% of respondents forecasting a rise in these technologies (see graph below).
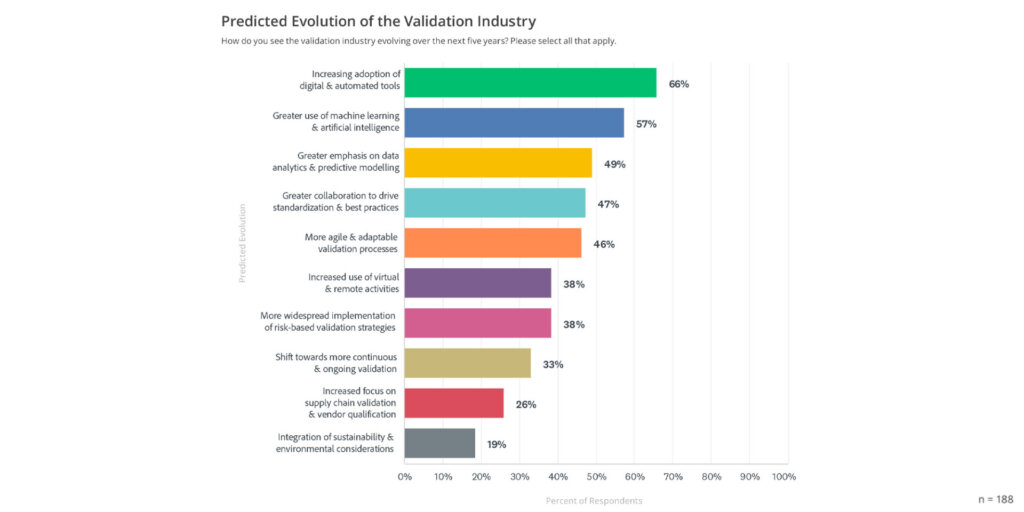
Digital tools enable more efficient workflows, reduce manual tasks, and streamline compliance processes, enhancing the speed and accuracy of validation. The report emphasizes that automated solutions not only improve productivity but also help organizations maintain robust data integrity and ensure continuous audit readiness.
3. Artificial Intelligence and Machine Learning
More than half (57%) of the respondents believe that AI and machine learning will become integral to validation. These technologies can handle large datasets, perform predictive modeling, and analyze patterns that may otherwise go unnoticed. Machine learning can also automate repetitive tasks and enhance decision-making by identifying potential risks earlier in the process.
On-Demand Webinar: Powering Process Validation Through AI
Alyssa Burke of BW Design Group and Joe Azzarella of Kneat show you why AI is the power-up your process validation needs and how to unlock it.
Watch to learn:
- The benefits and barriers to implementing Pharma 4.0 technologies, such as AI
- How validation professionals can use AI and machine learning in their processes
- How model efficacy, re-training models, and model validation can be influenced by AI
- What intelligent technologies mean for Stage 3 Process Validation
4. Enhanced Data Analytics
Nearly half of the surveyed professionals highlight data analytics and predictive modeling as key elements in the future of validation. This focus on data analytics reflects the industry’s shift towards proactive and data-driven validation processes.
As companies look to optimize their validation strategies, tools that offer predictive insights are invaluable in managing risk, improving compliance, and enabling faster, more informed decision-making.
5. Greater Collaboration and Standardization
Collaboration across departments, industries, and even geographical boundaries is expected to grow, with 47% of respondents anticipating increased information-sharing to drive standardization and best practices.
Digital validation platforms like Kneat Gx that facilitate real-time collaboration will play a significant role here, as they enable stakeholders to share data, insights, and validation strategies efficiently, fostering a more unified approach to validation.
6. Agile and Adaptable Validation Processes
Adaptability is essential in an industry as dynamic as life sciences, where regulatory requirements and technological advancements continuously evolve. The report notes 46% of respondents expect more agile and adaptable validation processes, allowing companies to quickly respond to changes and maintain compliance. This flexibility is crucial for organizations seeking to remain competitive and innovative.
7. The Rise of Remote and Virtual Validation
Remote and virtual validation are becoming more common, with 38% of participants indicating an increased reliance on these methods. This trend is likely driven by the rise of remote work and virtual collaboration technologies.
By leveraging tools like digital validation platforms, virtual reality (VR), and augmented reality (AR), organizations can conduct validation activities remotely, and facilitate remote regulatory assessments, audits, and inspections, which are on the rise.
During the COVID-19 pandemic, the U.S. Food and Drug Administration (FDA) needed to adapt to health recommendations like facility closures and social distancing while still overseeing the production of food and drug products the public used daily. The solution was Remote Regulatory Assessments (RRAs). In late 2023, the FDA announced it will be continuing its use of RRAs beyond the pandemic and will be expanding their use.
8. Continuous Validation Practices
Continuous validation is an emerging practice, with 33% of the respondents noting a shift in this direction. This approach ensures that validation is integrated throughout the product lifecycle, allowing for continuous monitoring and real-time updates. Continuous validation is especially relevant in environments with frequent updates or regulatory changes, helping organizations maintain consistent compliance and quality.
Future Validation Challenges
The 2024 State of Validation Report highlights a transformative period for validation, driven by digital innovation, collaboration, and a focus on adaptability. As organizations embrace these trends, they face challenges such as cost and resource constraints, the need for skilled professionals, and the rapid pace of technological change (see graph below).
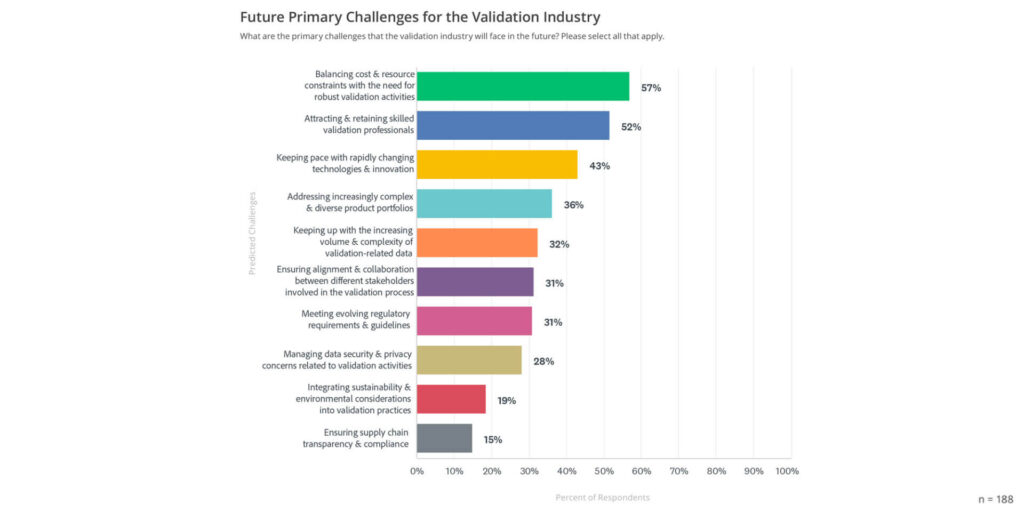
By adopting digital tools and fostering a culture of continuous improvement, organizations can build resilient validation processes that ensure compliance, improve efficiency, and ultimately contribute to safer, more reliable products.
Download the Full Report to Stay Ahead in 2025
To dive deeper into these insights and discover how the latest trends and technologies are shaping the future of validation, download the full 2024 State of Validation Report today. Get access to detailed data, expert analyses, and practical recommendations that can help you optimize your validation processes and stay ahead in 2025.







