Easily track parameter change
Kneat’s "Entities" feature ensures method parameters are automatically reproduced across documents. Enabling accurate, real-time tracking of changes with full traceability, enhancing compliance and consistency.
Address analytical challenges
Unique testing scenarios introduce risks. Kneat streamlines the management of matrix effects and variability, ensuring methods remain robust and compliant.
Digital first
Kneat’s digital approach goes beyond paper to glass, enhancing manual method validation workflows, improving efficiency, and increasing accuracy while aligning with regulatory expectations.
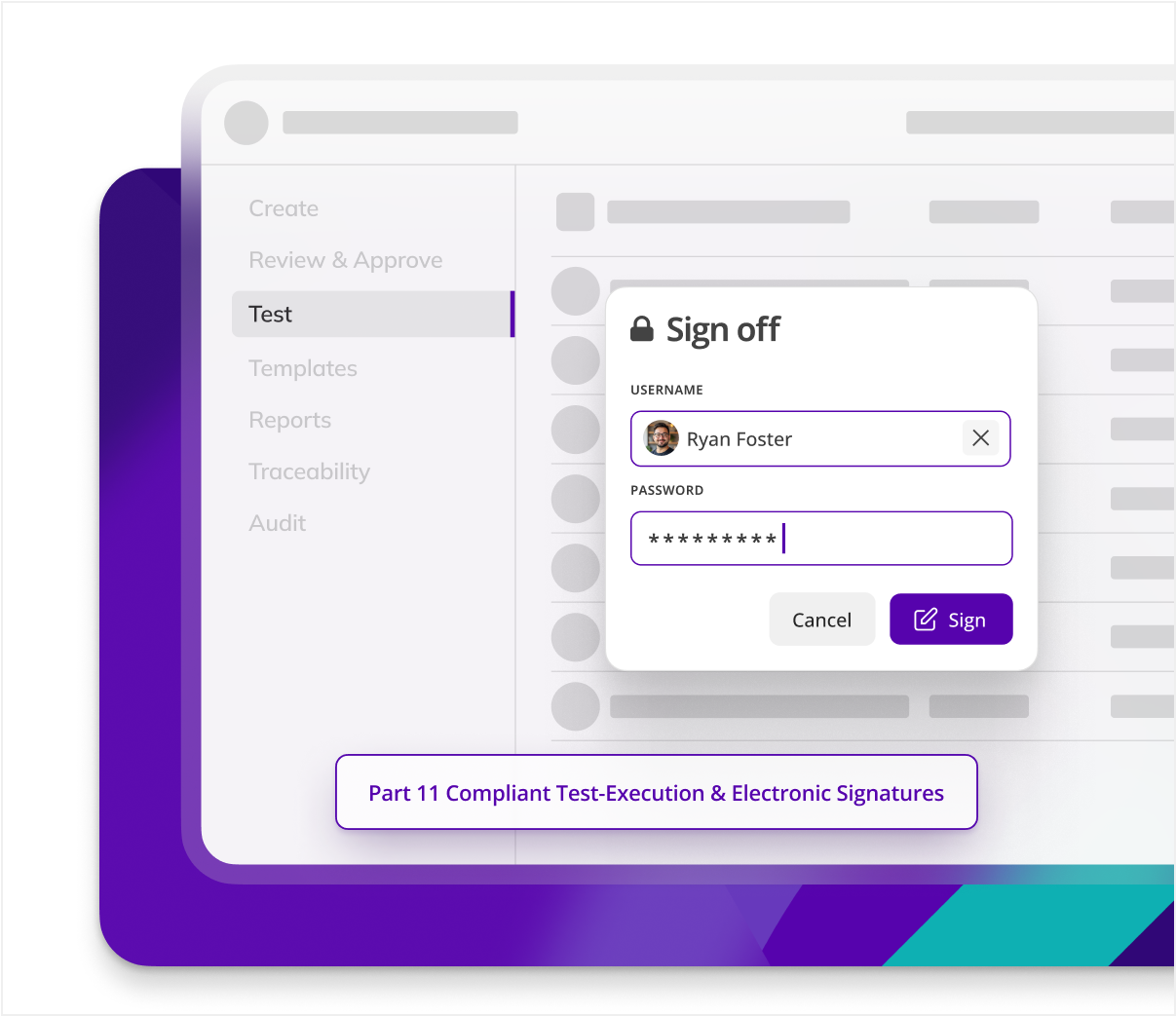
Protect data integrity
Data integrity risks arise during manual transcription of results in method validation. Kneat eliminates this by digitizing data entry and ensuring audit trails. Safeguarding compliance and accuracy throughout the validation process.
Trusted By
Customer Success
Case studies

We were able to demonstrate over 50% cycle time reduction…and process simplification from 15 steps to 8 … because of Kneat we minimized the number of systems we used from 5 to 2.
- Global Executive Director, MSD
Book a demoResults
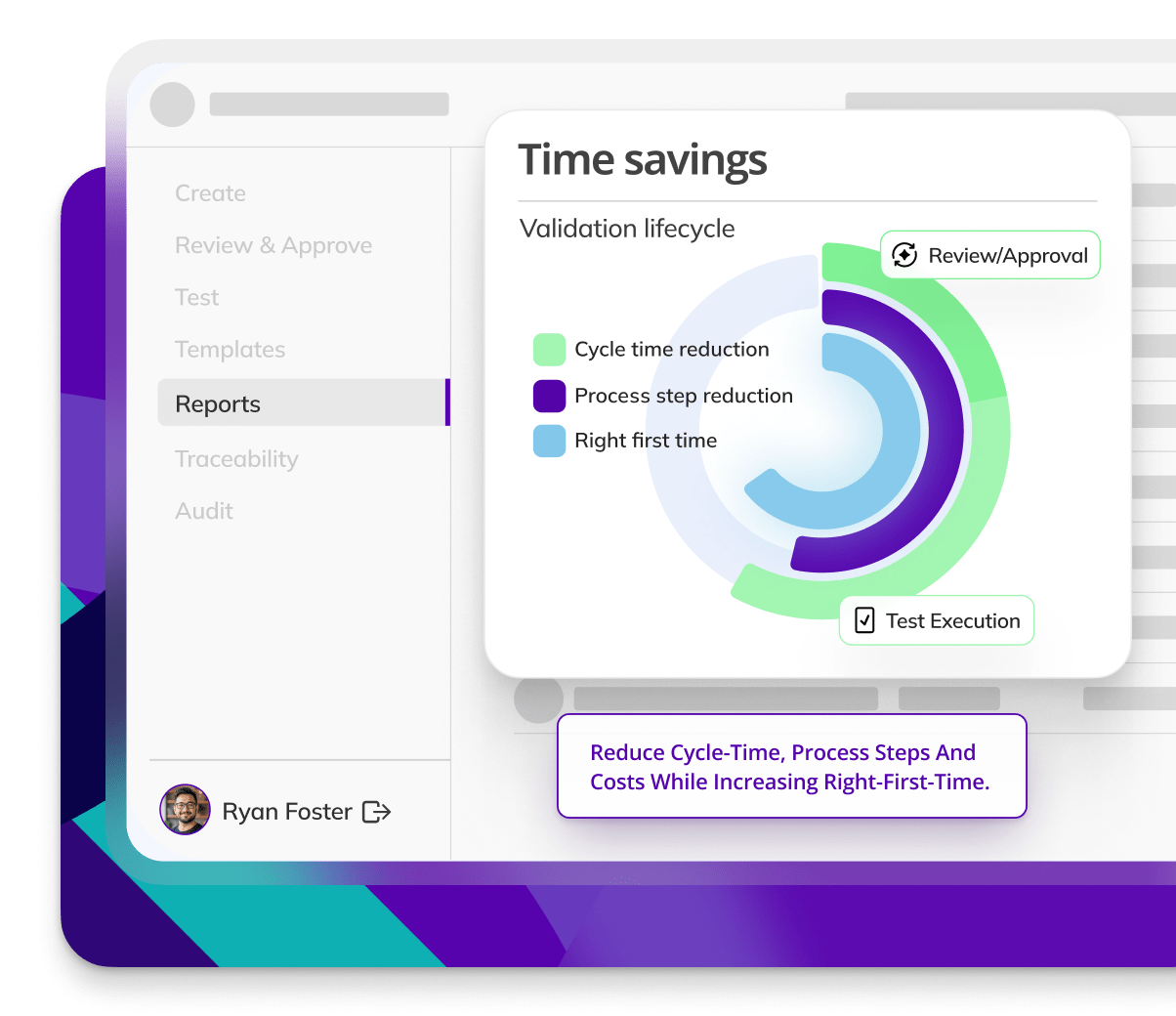
CYCLE TIME REDUCTION
Kneat is proven to reduce cycle time by an average of 50% or more in multiple customer authored case studies.
CSAT Score
97% of our customers rate our customer support as either ‘Very Good’ or ‘Excellent.’
PLAN TO RENEW
Customer feedback via industry analyst SoftwareReviews.
Revolutionize your validation
Digitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Book a demo