Electronic logbook management software
Kneat’s eLogbooks is a purpose-built digital solution for pharma, medical devices, and other highly regulated industries. Ensure regulatory compliance and inspection-ready records.
Book a demoSolving GxP logbook challenges
Log anything
Log any process — from equipment use and maintenance to cleaning and deviation tracking. Tailor your logs to fit any need.
Legible, compliant logs
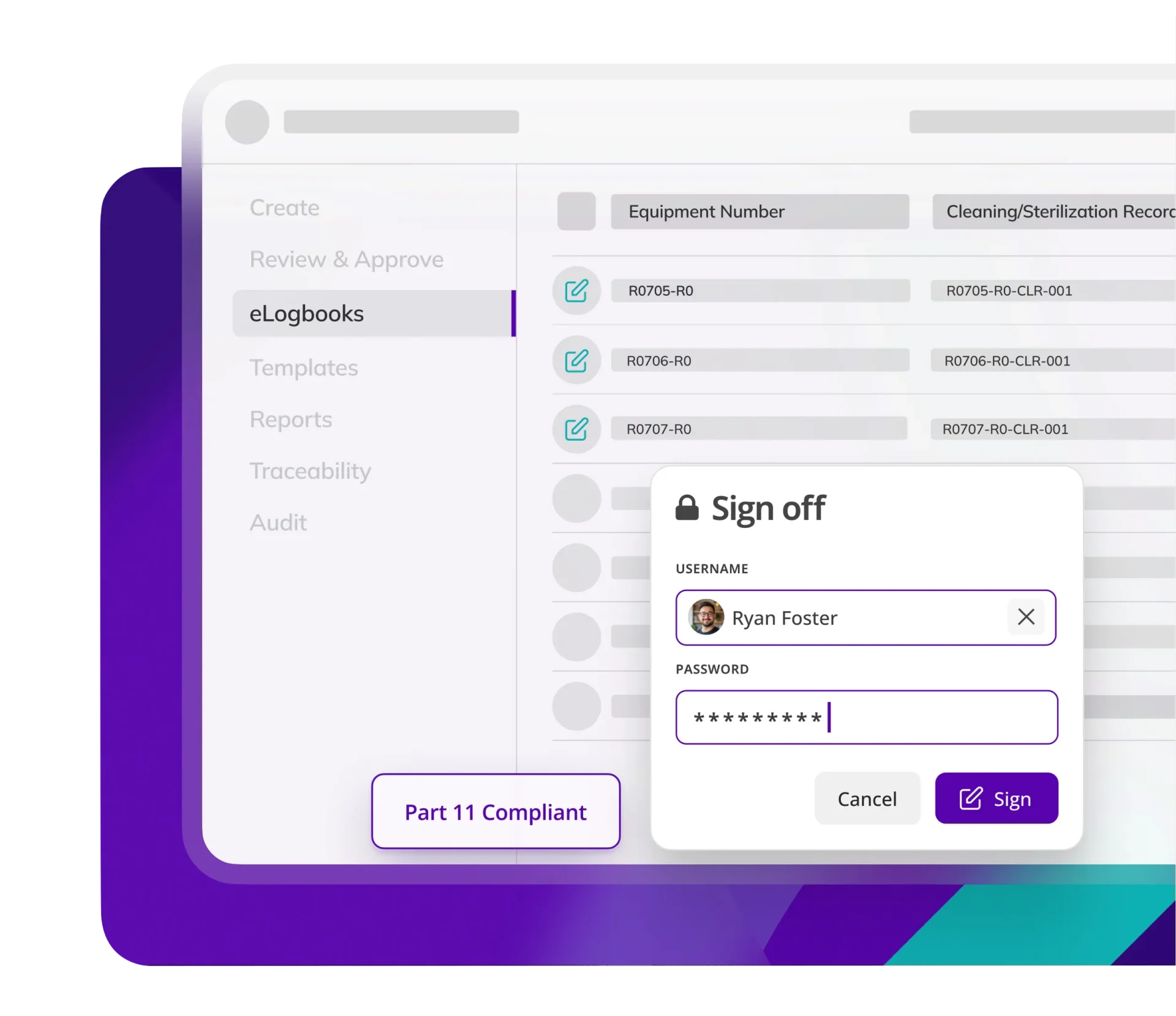
Kneat ensures log entries are legible and traceable and meet ALCOA++ standards. Reducing risk and assuring logbook data integrity.
Easy to use and access
Kneat’s user-friendly interface makes logbook management fast and easy for both regular and new users, such as production operators or technicians.
Secure virtual auditor area
Use Kneat Gx to house post-approved eLogbooks in an auditor staging area to seamlessly respond to auditor's requests.
Trusted By

Now, you sign into Kneat, sign the row, and you’re essentially done issuing the logbook. I don’t have to spend time creating the logbook or archiving the logbook. It’s all in one place
- Kailash Rathi, Director, Quality Systems & Validation – ReciBioPharm
Book a demoCustomer Success
Case studies
Results
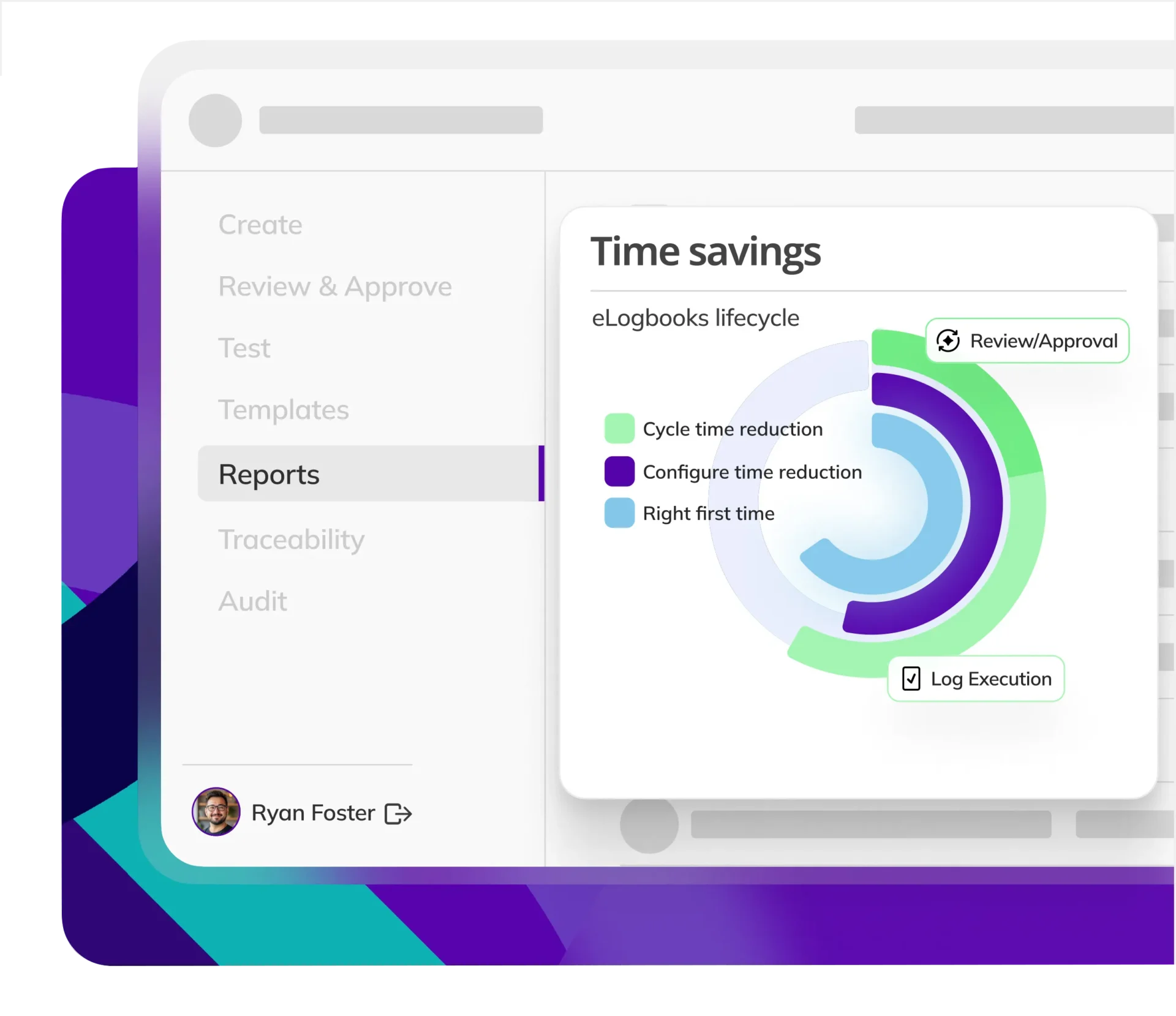
CYCLE TIME REDUCTION
Kneat is proven to reduce cycle time by an average of 50% or more in multiple customer authored case studies.
CSAT Score
97% of our customers rate our customer support as either ‘Very Good’ or ‘Excellent.’
PLAN TO RENEW
Customer feedback via industry analyst SoftwareReviews.
What is Kneat eLogbooks and how does it improve traditional logbook processes?
Kneat eLogbooks are a digital, compliant alternative to paper-based logbooks, designed for regulated industries. It ensures real-time, traceable, and audit-ready logging for equipment use, maintenance, cleaning, calibration, and more while reducing errors, eliminating paper records.
How does Kneat eLogbooks support regulatory compliance like 21 CFR Part 11?
Kneat eLogbooks include built-in support for electronic signatures, full audit trails, role-based permissions, and ALCOA++ data integrity principles. Every log entry is traceable, time-stamped, and securely stored, helping companies meet FDA and global regulatory standards.
Can Kneat eLogbooks be customized for different types of logs and workflows?
Yes. Kneat eLogbooks are fully configurable for organizations to design log templates for equipment use, environmental monitoring, deviations, sanitation, and more. Templates and approval workflows can be tailored by site, department, or equipment type.
What are the benefits of integrating Kneat eLogbooks with equipment lifecycle and validation activities?
Kneat eLogbooks allow you to link log entries to the entire equipment lifecycle, including installation, qualification, or decommissioning. This provides end-to-end traceability across validation, maintenance, and compliance data, supporting faster audits and better decision-making.
Who typically uses Kneat eLogbooks and what industries is it built for?
Kneat eLogbooks are purpose-built for regulated life sciences sectors such as pharmaceuticals, biotechnology, medical devices, and CMOs where precise, compliant, and traceable documentation is essential for operations, audits, and inspections.
Revolutionize your validation
Digitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Book a demo