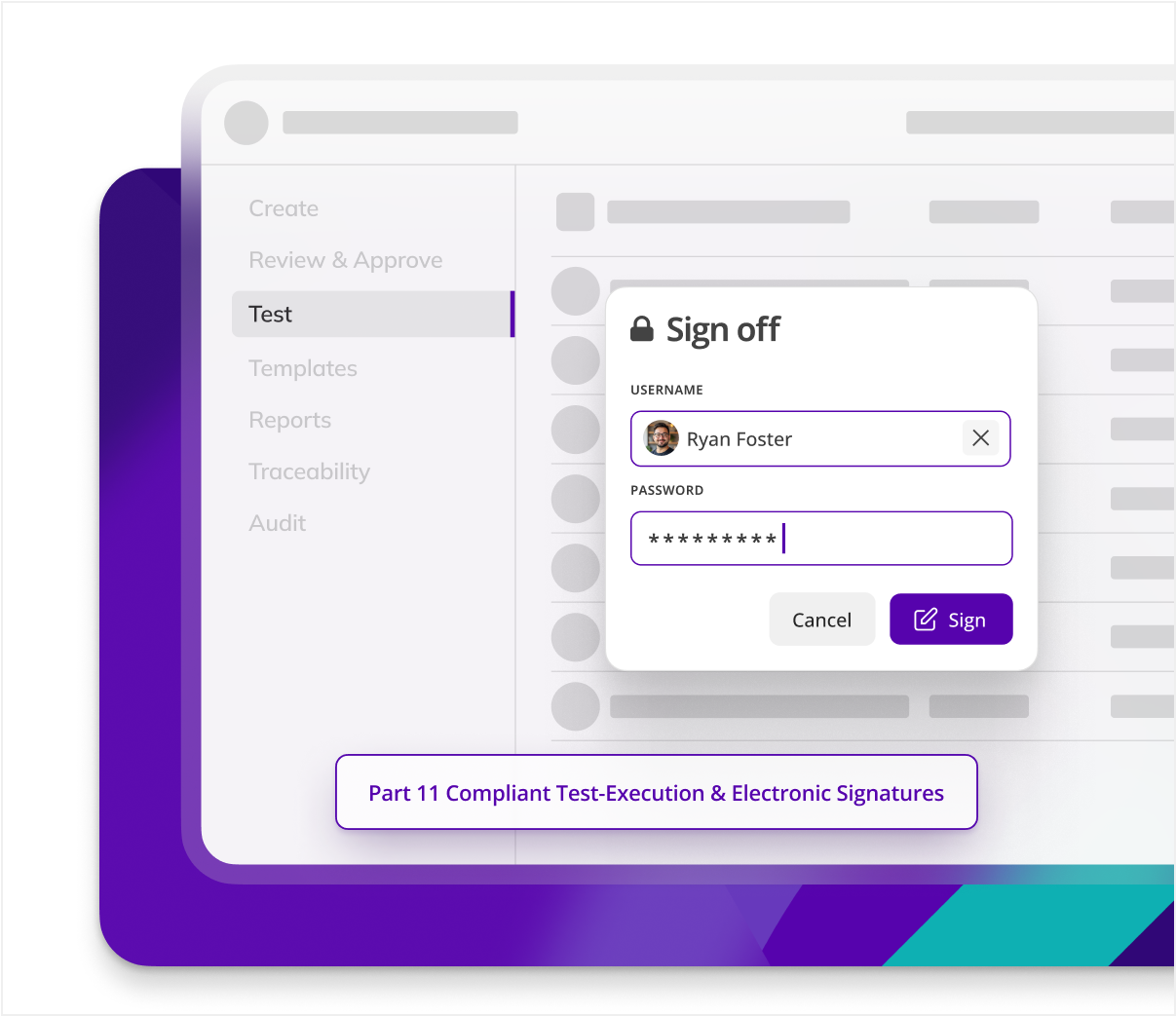
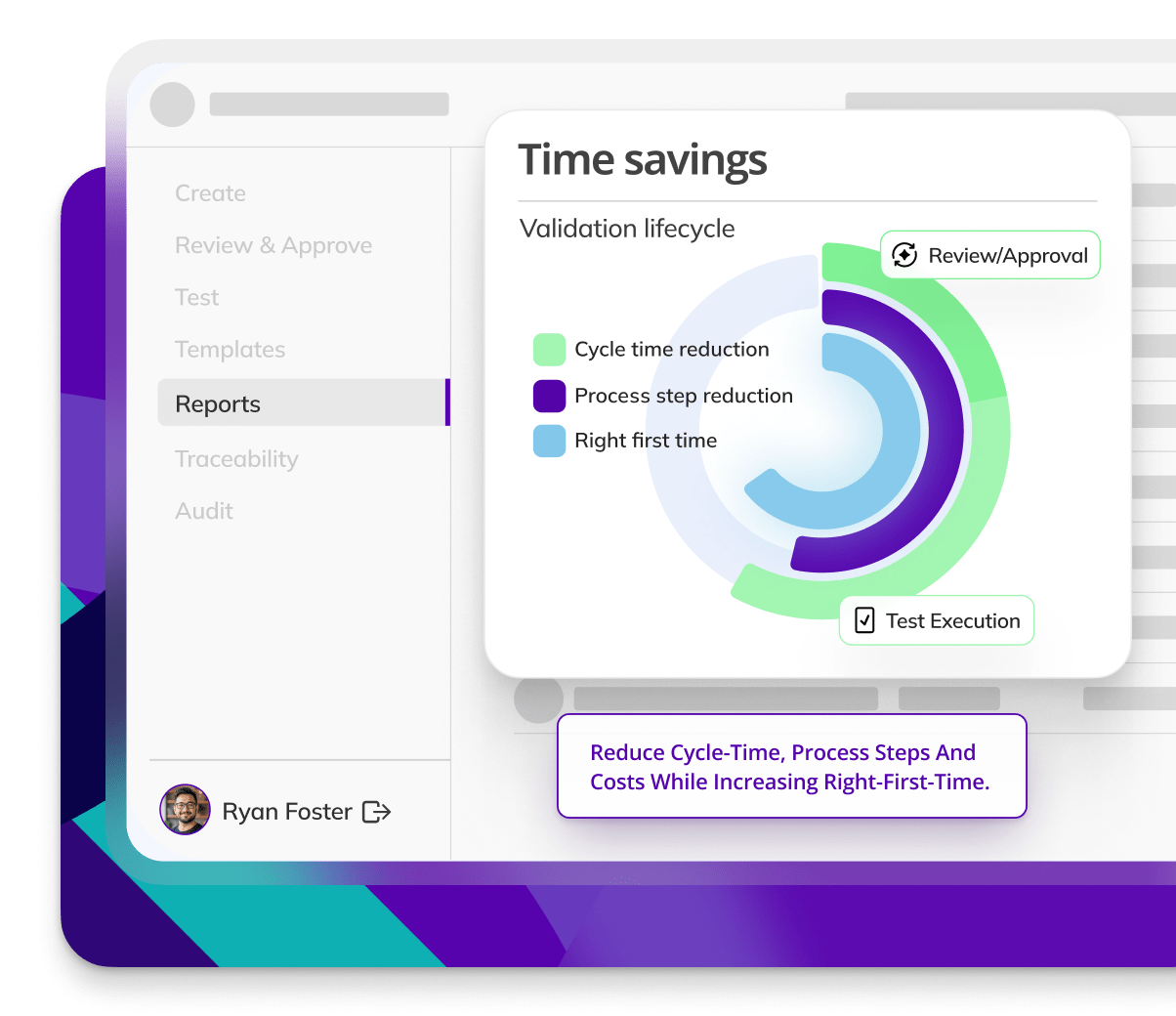
Enhance compliance and efficiency
Kneat digitalizes the entire commissioning & qualification (C&Q) validation lifecycle. Delivering significant productivity improvements and assuring compliance with regulatory standards.
Implement risk-based approaches
Kneat facilitates a risk-based, lean, lifecycle approach to C&Q that is aligned with ASTM E2500 best practice methods.
Eliminate paper records
By digitalizing C&Q processes, Kneat eliminates 100% of paper records, reducing physical storage needs and associated costs, while also minimizing protocol-based GDP errors.
Gain real-time process visibility
Kneat provides instant macro and micro visibility into all aspects of the C&Q process in real time, enabling better decision-making and proactive issue resolution.
Trusted By
Customer Success
Case studies

Before, we had Veeva....We had a lot of documentation, but it did not have the level of organization and accessibility that we have with Kneat. We also couldn’t execute C&Q digitally.
- Global Director, Commissioning & Qualification
Book a demoResults
MORE EFFICIENT FOR c&Q THAN COMPETITOR
Kneat is proven to be 40% more efficient for commissioning & qualification than a leading competitor product. As found in a customer comparative pilot study.
urs approval cycle time reduction
Kneat is proven to reduce user requirements specification approval cycle time by 88%. As found in a customer authored case study.
Reduction in Review-Approval Time
Kneat is proven to reduce review-approval cycle time by 40%. As found in a customer authored case study.
Revolutionize your validation
Digitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Book a demoDoes Kneat automate test script generation and execution for commissioning?
Yes. Kneat provides digital test script authoring, execution, and review workflows. This automation reduces manual effort, eliminates paper-based errors, and accelerates commissioning timelines by allowing teams to execute IQ/OQ/PQ and commissioning tests directly within the platform.
Can Kneat support the full lifecycle of qualification (IQ, OQ, PQ)?
Absolutely. Kneat supports Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) protocols in a single, unified environment. This ensures end-to-end traceability across the entire validation lifecycle — from commissioning through ongoing qualification.
How does Kneat handle change control and deviations?
Kneat includes built-in workflows for documenting, tracking, and resolving deviations and change controls. Every action is recorded in a secure audit trail, making it simple to demonstrate compliance during inspections.
Does Kneat align with risk-based approaches like ASTM E2500 and ICH Q9?
Yes. Kneat is designed for risk-based validation strategies. It enables users to build risk assessments, link risks to protocols, and implement a science- and risk-based approach in line with ASTM E2500 and ICH Q9 expectations.
Does Kneat offer version control for validation documents?
Yes. All validation documents in Kneat are version controlled. Edits, approvals, and updates are tracked with full visibility, ensuring teams always know the current approved version.
Are there customizable templates for validation deliverables?
Yes. Kneat provides configurable templates for validation plans, protocols, traceability matrices, and reports. This avoids the need to start from scratch and ensures consistency across projects and sites.