Validation Use Cases
Cleaning validation software
Enforced standards, secure compliance, digital validation makes products clean andsafe, every time.
Book a demoTrusted By
Customer Success
Case studies

We were able to demonstrate over 50% cycle time reduction…and process simplification from 15 steps to 8 … because of Kneat we minimized the number of systems we used from 5 to 2.
- Global Executive Director, MSD
Book a demoResults
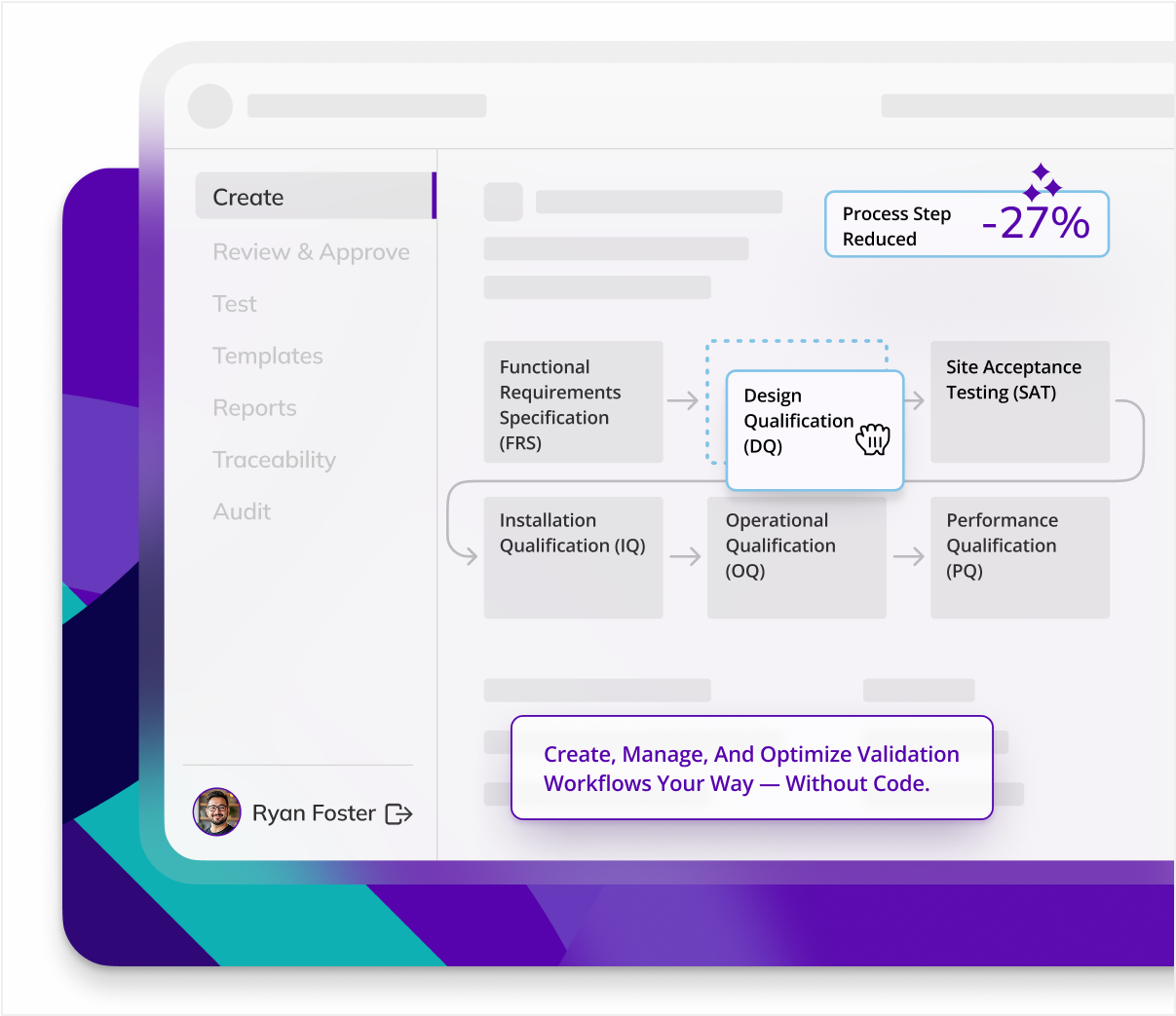
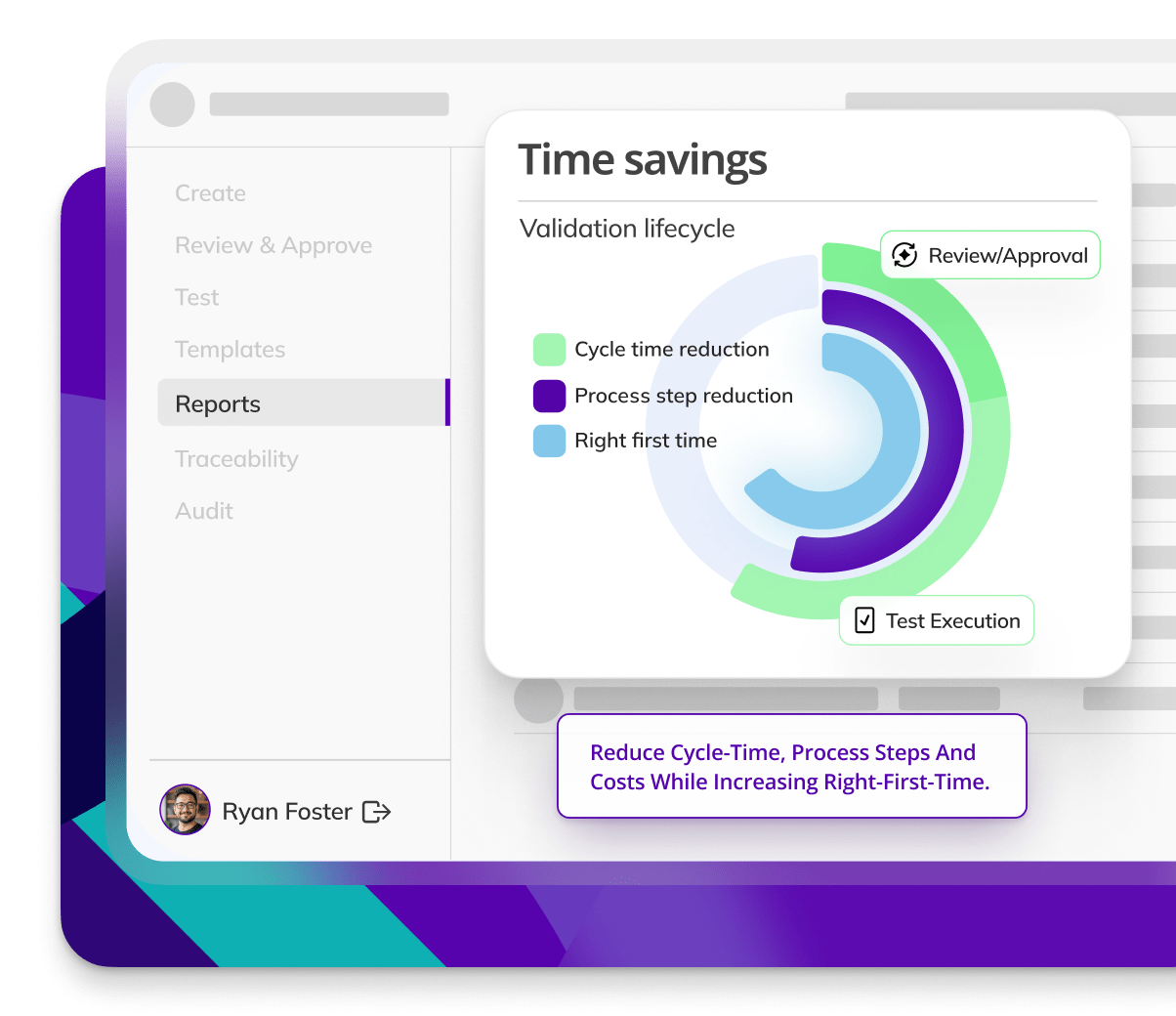
CYCLE TIME REDUCTION
Kneat is proven to reduce cycle time by an average of 50% or more in multiple customer authored case studies.
CSAT Score
97% of our customers rate our customer support as either ‘Very Good’ or ‘Excellent.’
PLAN TO RENEW
Customer feedback via industry analyst SoftwareReviews.
Revolutionize your validation
Digitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Book a demoDoes Kneat meet 2025 lifecycle validation expectations (ALCOA⁺, 21 CFR Part 11 / Annex 11) and provide audit-ready protocol/report packs?
Kneat Gx is 21 CFR Part 11 / Annex 11 compliant and built around ALCOA++ data-integrity principles, and the product includes audit trails and a Collections feature for secure, fully digital audit handover. Kneat also makes template management easy and lets your team author IQ/OQ/PQ and other validation documents inside the platform so they’re always audit ready.
Does Kneat ship built-in, audit-ready templates (IQ/OQ/PQ, CVP, MACO calculations) and an automated change-control workflow that stays evergreen?
Kneat supports template management, preloaded template packs and automated workflows to accelerate protocol creation and approvals, and the platform allows authoring/locking of validation documents (IQ/OQ/PQ, risk assessments, change controls) within the system. Kneat also is flexible and configurable, so your validation teams can create the documents and processes they need, no matter the requirement.