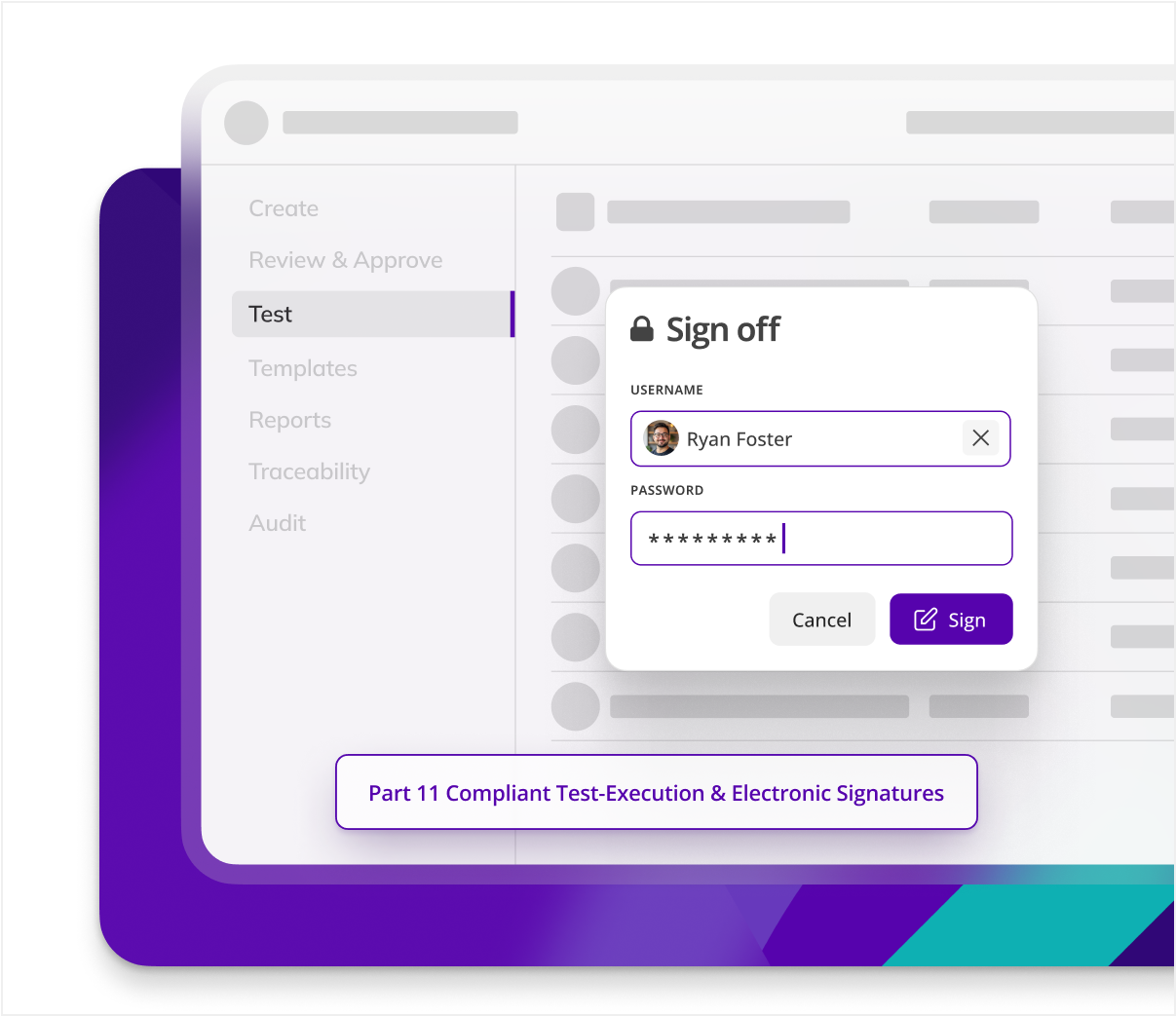
Assure accuracy
Kneat digitalizes the entire analytical instrument validation lifecycle, from user requirements to final reports, ensuring instruments consistently deliver accurate results.
Streamline documentation
With pre-approved templates and dynamic data sharing, Kneat simplifies document creation, reducing time and minimizing errors by eliminating manual data transposition.
Unparalleled data integrity
Kneat delivers unparalleled ALCOA++ data integrity control for any validation process, reducing GDP errors and improving data integrity throughout the validation process.
Standardize instrument validation
Kneat supports global harmonization and standardization, reducing deployment time for new instruments and minimizing downtime during changes or upgrades.
Trusted By
Customer Success
Case studies

Before, we had Veeva....We had a lot of documentation, but it did not have the level of organization and accessibility that we have with Kneat. We also couldn’t execute C&Q digitally.
- Global Director, Commissioning & Qualification
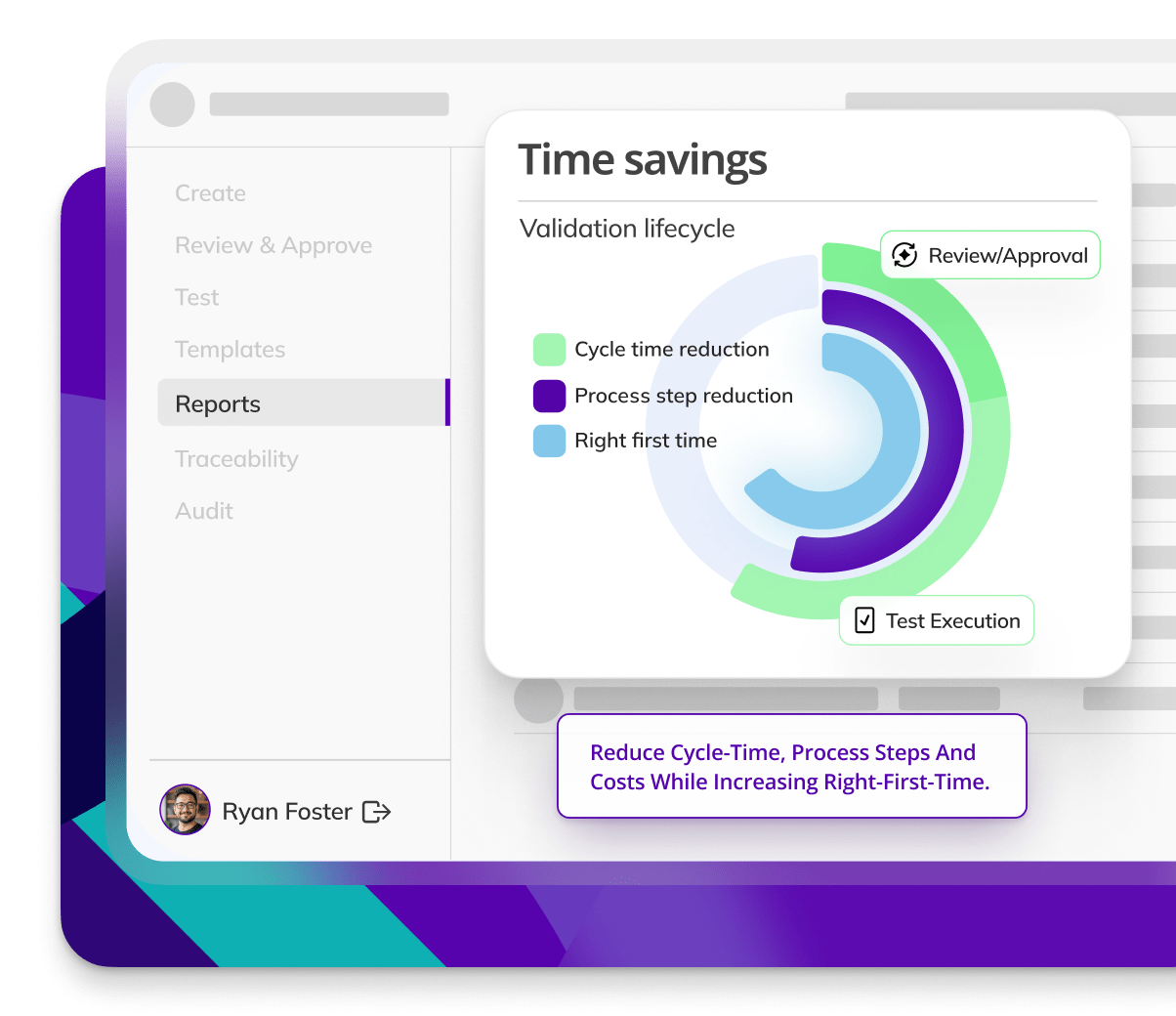
Book a demoResults
CYCLE TIME REDUCTION
Kneat is proven to reduce cycle time by an average of 50% or more in multiple customer authored case studies.
CSAT Score
97% of our customers rate our customer support as either ‘Very Good’ or ‘Excellent.’
PLAN TO RENEW
Customer feedback via industry analyst SoftwareReviews.
Revolutionize your validation
Digitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Book a demo