Minimize downtime
Validation downtime impacts production. Kneat automates scheduling, minimizing disruption and maintaining operational efficiency during validation processes.
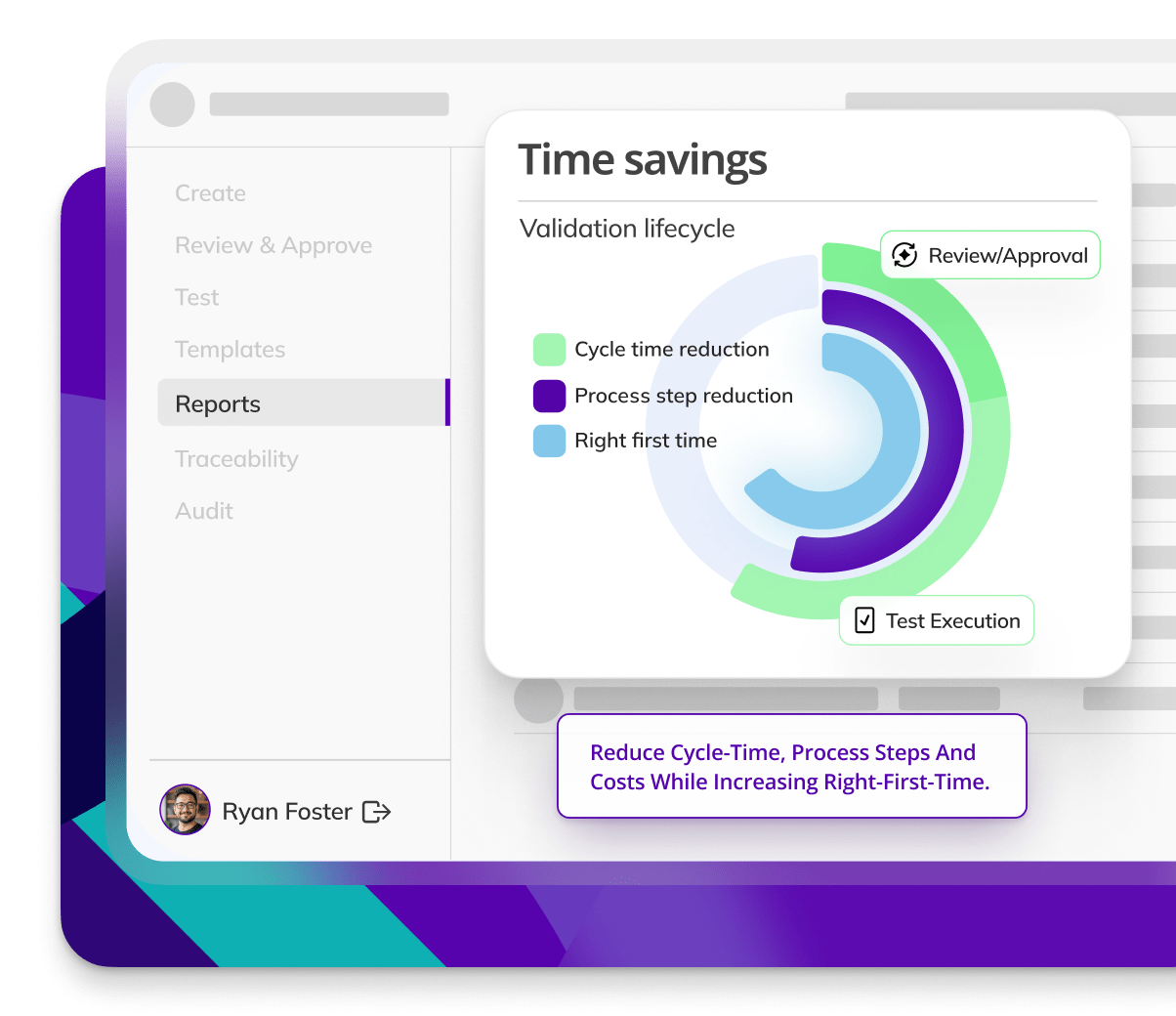
Track performance consistently
Performance consistency is hard to track. Kneat provides real-time insights into equipment performance, assuring ongoing compliance with validation standards.
Manage equipment upgrades
Obsolescence creates risk. Kneat helps manage equipment upgrades and validations seamlessly, keeping systems up-to-date without compromising compliance.
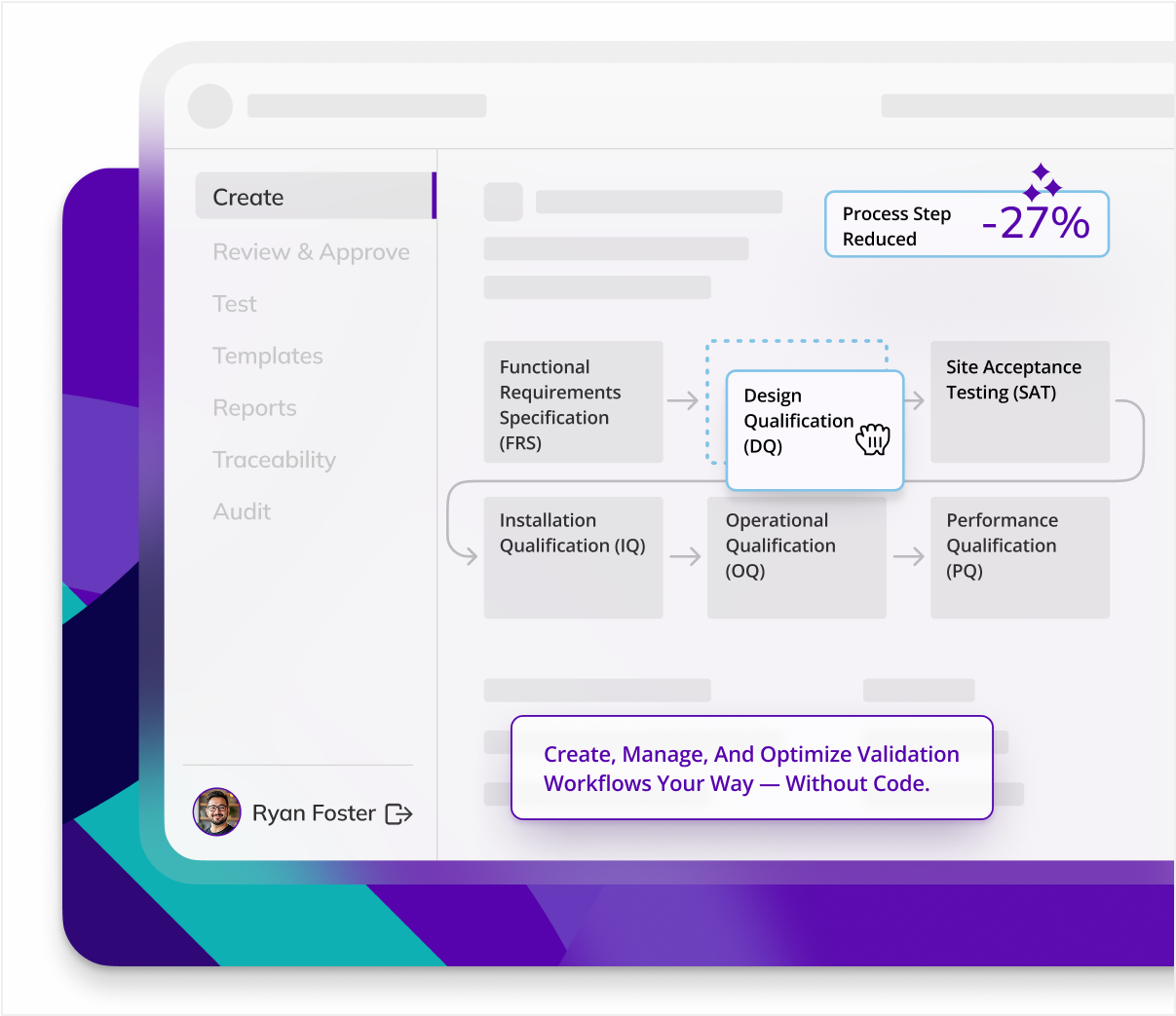
Standardize across sites
Multi-site equipment validation can be complex, but Kneat standardizes validation processes across locations. Use Kneat to assure uniform compliance across all facilities.
Trusted By
Customer Success
Case studies

We were able to demonstrate over 50% cycle time reduction…and process simplification from 15 steps to 8 … because of Kneat we minimized the number of systems we used from 5 to 2.
- Global Executive Director, MSD
Book a demoResults
MORE EFFICIENT FOR c&Q THAN COMPETITOR
Kneat is proven to be 40% more efficient for commissioning & qualification than a leading competitor product. As found in a customer comparative pilot study.
urs approval cycle time reduction
Kneat is proven to reduce user requirements specification approval cycle time by 88%. As found in a customer authored case study.
Reduction in Review-Approval Time
Kneat is proven to reduce review-approval cycle time by 40%. As found in a customer authored case study.
Revolutionize your validation
Digitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Talk to an expert