At Kneat, we’ve long believed that digital validation tools (DVTs) are the cornerstone of a more agile, compliant, and efficient life sciences industry. That belief has just been reinforced by the 2025 release of the ISPE® Good Practice Guide: Digital Validation. This comprehensive document is the industry’s first formalized framework for implementing DVTs — and it’s a landmark moment for validation professionals worldwide.
As a company founded to meet the technical demands of regulated validation, Kneat is proud to have contributed to the growing maturity of this space. Our CEO, Eddie Ryan, brings deep validation expertise to the helm. A mechanical engineer by training, Eddie has spent his career optimizing compliance in regulated manufacturing environments —experiences that led directly to the creation of Kneat’s digital validation platform. Today, Kneat Gx is used by some of the world’s largest life sciences companies to standardize, automate, and transform their validation processes.
Why the ISPE Good Practice Guide on DVTs matters now
The ISPE Guide is timely: according to a 2023 survey conducted by the ISPE C&Q Community of Practice, 75% of companies have implemented or plan to implement DVTs within two years. However, many still struggle to scale or govern these systems effectively.
Data from the 2025 State of Validation report reinforces this trend.
Challenges During Digital Validation Implementation
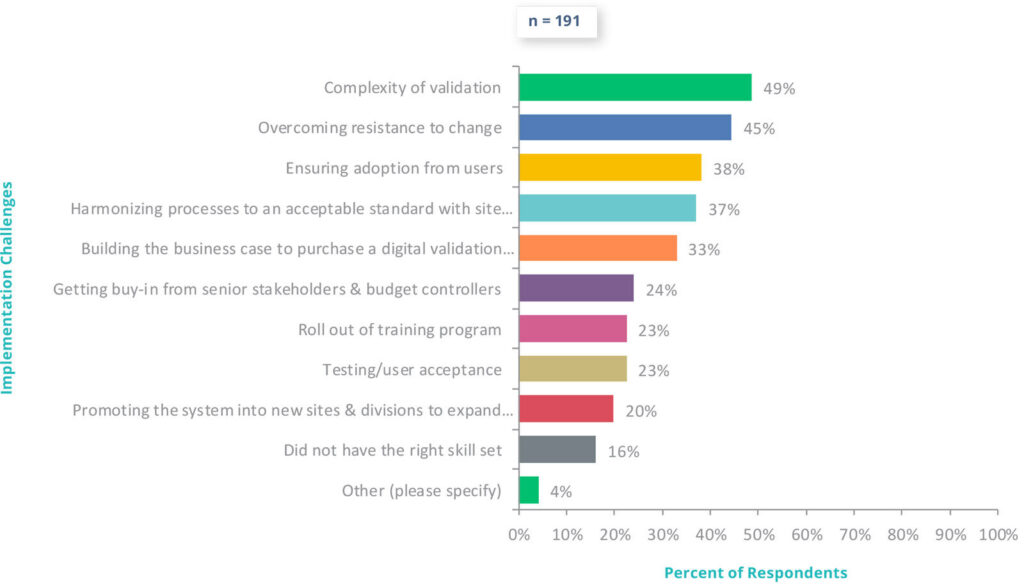
- Of those who say they have already adopted or are currently adopting a digital validation system, nearly half of all respondents (49%) identified the complexity of validation itself as the top implementation challenge, underscoring the need for systems that can handle diverse regulatory requirements and risk levels.
- Overcoming resistance to change was the second most common challenge, highlighting the cultural and behavioral hurdles that can slow digital adoption — even with the right technology in place.
- Close to 40% of respondents struggled with ensuring adoption from users, pointing to the importance of intuitive design, proper onboarding, and effective training.
The gap between initial deployment and full digital maturity remains a key hurdle for many organizations. The Guide is designed to help companies bridge that gap. It provides practical, risk-based best practices for selecting, implementing, and governing DVTs in regulated environments. It’s not a regulatory standard, but it aligns with ISPE GAMP® 5 (Second Edition), CSA principles, and data integrity guidance like ALCOA++ — offering regulated companies the confidence to move forward.
Key concepts and takeaways
- From paper-on-glass to Validation 4.0
The Guide makes a clear distinction between digitizing paper processes (“paper-on-glass”) and implementing true digital transformation. DVTs should not just mimic paper — they should improve on it by automating traceability, enabling collaboration, integrating systems, and producing actionable insights. This is central to the vision of Validation 4.0: a future where validation is risk-based, iterative, and seamlessly integrated across the product lifecycle.
Read our eBook Validation 4.0 in Life Sciencesto explore how Validation 4.0 complements Pharma 4.0, creating efficiencies previously unattainable. This eBook delves into transformative technologies like blockchain, IoT, and digital validation, illustrating their application in top-tier validation processes.
- The business case for DVTs
Validation leaders are often asked to justify the transition from paper to digital. The Guide provides a strong framework for building that case, detailing clear ROI areas such as:
- Reduction in review and approval cycle times
- Enhanced data integrity through audit trails and secure access
- Remote execution and review capabilities
- Elimination of paper storage costs
- Standardized templates for global harmonization
For those already using Kneat Gx, these benefits will sound familiar — they’re exactly what we deliver every day for our customers.
- Data integrity by design
The Guide dedicates significant attention to data integrity — an area Kneat has championed for years. It outlines how DVTs can fulfill ALCOA++ principles and enable “true copy” strategies that allow organizations to move away from managing physical documentation altogether. This not only improves compliance but also accelerates audits and inspections by making data searchable, complete, and available in real time.
- Governance is critical
Chapter 6 of the Guide stands out for its in-depth guidance on governance. It advises a balanced top-down and bottom-up approach, supported by a formal governance structure that includes quality, IT, validation, and process owners. It stresses the importance of training, change control, and continuous monitoring of KPIs to maintain the validated state.
- Integration: the digital ecosystem vision
Perhaps the most ambitious part of the Guide is its call for integration. It envisions a connected digital validation ecosystem where DVTs interact seamlessly with MES, LIMS, ERP, and more. The goal is a data-centric environment where insights flow freely across platforms to support quality decisions.
Kneat Gx flexes to allow you to create any validation process, your way — anytime — within one complete solution. You can choose a document or data-centric approach and enjoy the flexibility to build and manage your process flow the way you require, whatever your validation methodology.
Cultural change: the human factor
The Guide rightly emphasizes that digital transformation is as much about people as it is about technology. Successful DVT adoption requires a mindset shift — from risk aversion to risk management, from silos to collaboration, and from static documents to dynamic data.
This aligns with what we’ve seen in successful Kneat Gx deployments: executive sponsorship, cross-functional ownership, and hands-on training are just as important as choosing the right tool.
That’s why we created Kneat Academy — a flexible, role-based training platform that empowers users to become confident and compliant digital validation practitioners from day one. Whether you’re new to digital validation or scaling across global teams, Kneat Academy ensures your people are ready.
Digital maturity assessment
The ISPE recommends incorporating a digital assessment into an organization’s digital validation strategy to align with wider goals and digital transformation objectives.
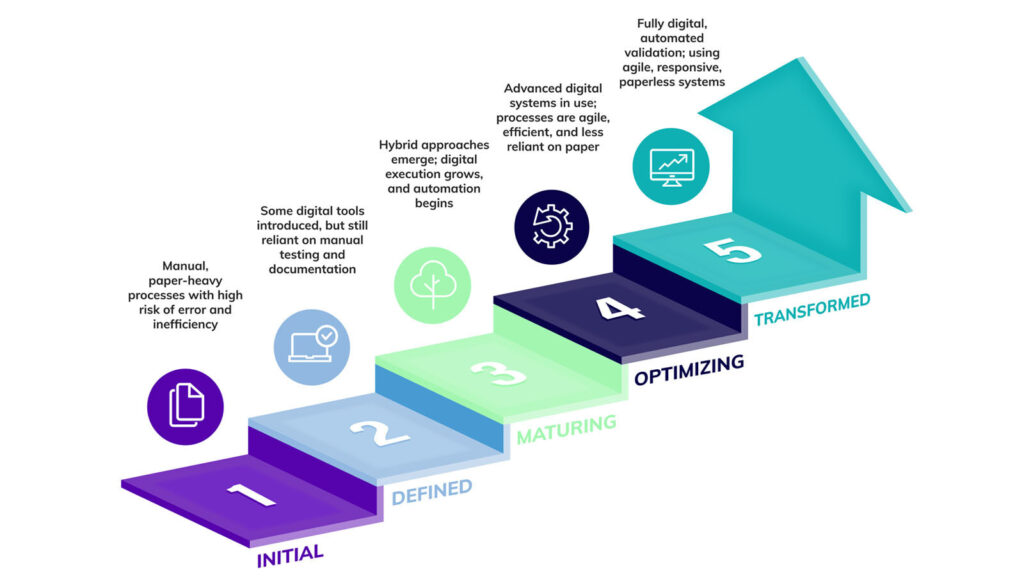
Kneat supports this approach through our own partner network and in-house consulting services, which help teams assess their digital validation maturity and evolve from Level 1 (“initial”), where organizations rely heavily on paper-based validation to Level 5 (“transformed”), where validation data is dynamic, traceable, and integrated across systems.
The ISPE’s Digital Validation Maturity Levels
Kneat’s commitment to the future of digital validation
As validation moves into its digital future, we’re excited to see ISPE provide the structure and guidance needed to scale adoption industry-wide.
For companies still navigating their transition, the ISPE Good Practice Guide: Digital Validation is a vital resource — and Kneat is here to help bring it to life.
Ready to go deeper?
Join our webinar, ISPE Digital Validation Guide: Expert Insights to explore key insights with Phil Jarvis and Dave O’Connor — co-authors of the ISPE Guide — and learn how to apply them effectively in your own validation programs. You’ll also see how Kneat helps you align with ISPE principles and best practice!






