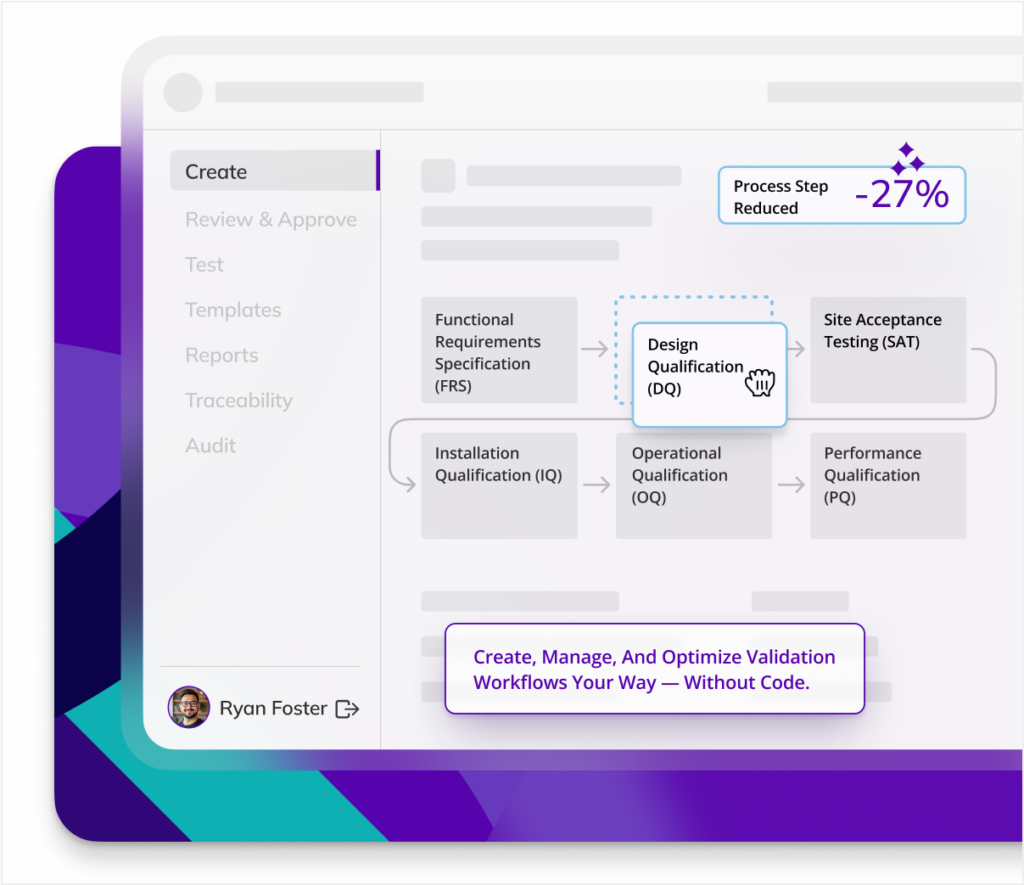
Flexible, compliant batch records
Kneat enables effortless batch record creation that adapts to your manufacturing needs, while ensuring compliance. Delivering a streamlined, reliable quality assurance process.
Assured batch data integrity
Kneat enforces ALCOA++ data integrity protections to production and other batch data. Assuring batch records are compliant, accurate, and meet both regulator and customer standards.
Improved traceability
Kneat centralizes validation and batch record data, providing clear traceability for customer and regulatory audits. Ensuring all documentation is easily accessible when needed.
Reduced errors & delays
Errors in batch records can lead to production delays, costly rework, and compliance risks. Kneat’s single solution integrates validation and batch record management. Eliminating mismatched data and manual errors.
Trusted By
Customer Success
Case studies

We were able to demonstrate over 50% cycle time reduction…and process simplification from 15 steps to 8 … because of Kneat we minimized the number of systems we used from 5 to 2.
- Global Executive Director, MSD
Book a demoResults
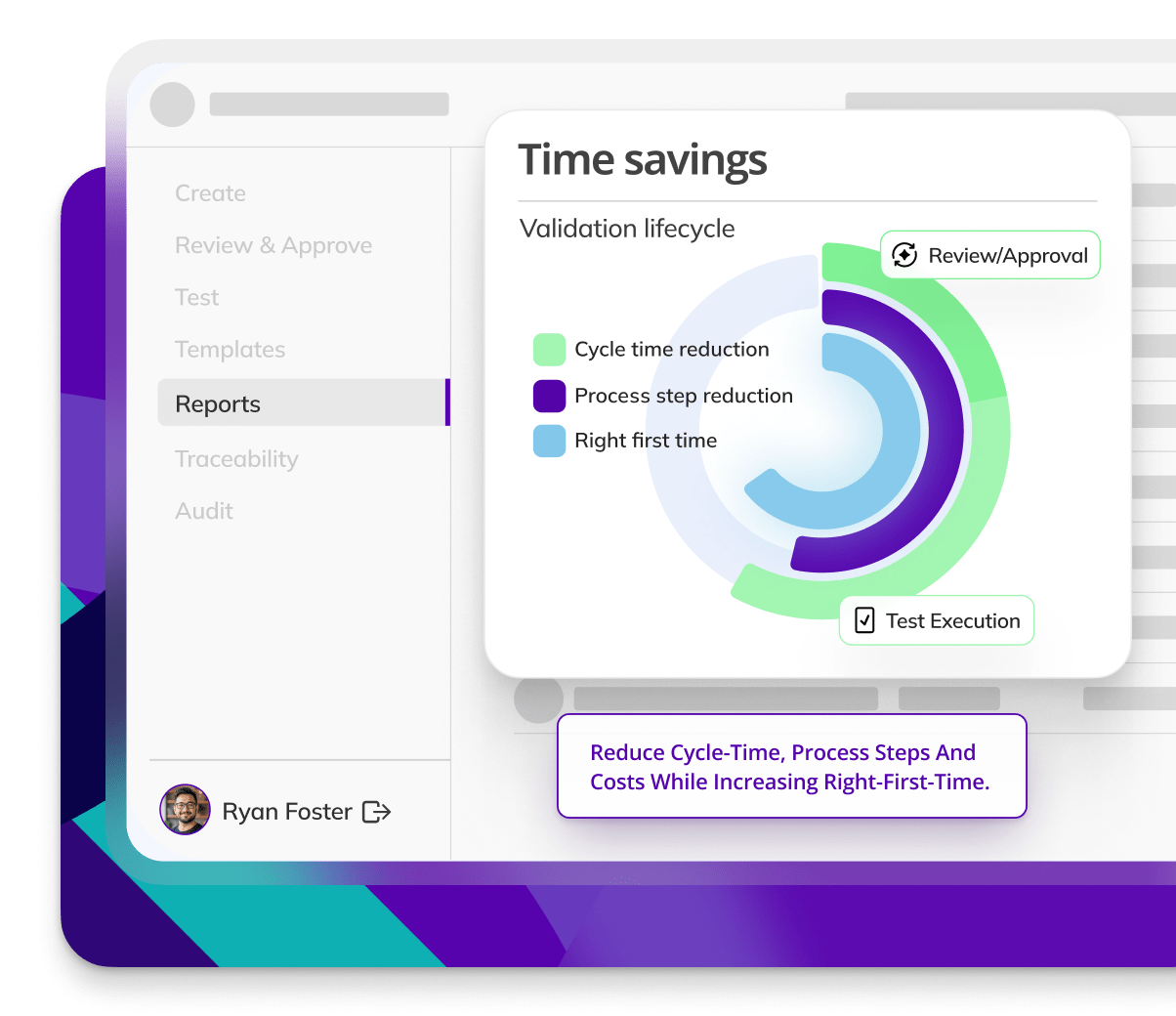
CYCLE TIME REDUCTION
Kneat is proven to reduce cycle time by an average of 50% or more in multiple customer authored case studies.
CSAT Score
97% of our customers rate our customer support as either ‘Very Good’ or ‘Excellent.’
PLAN TO RENEW
Customer feedback via industry analyst SoftwareReviews.
Revolutionize your validation
Digitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Book a demo