Validation Use Cases
Computer system validation (CSV) software
Regulators have highlighted paper-based validation as a potential CSV compliance pitfall. Learn how to build risk-based validation to protect data integrity and compliance with Kneat’s CSV software.
Talk to an expertTrusted By
Efficient change control
Frequent software updates and evolving business needs force validation teams into continuous revalidation cycles, straining resources and increasing risk. Kneat streamlines change control enabling you to reduce change-assessment turnaround and change-implementation time.
Ensure data integrity
Data integrity is often challenging, yet critical to maintain. Kneat enforces compliance through secure and traceable data integrity checks, safeguarding regulatory adherence.
Reach industry 4.0 faster
Kneat accelerates computer software assurance by reducing review-approval cycle times and supporting flexible, risk-based testing. Ensuring validation professionals keep pace with Industry 4.0’s expanding system landscape.
Remote freedom
Kneat enables 100% remote computer systems validation, reducing deployment time and costs for global systems.

It’s what I really wanted, the ability to still author and execute in the traditional manner, but not having the ‘on paper’ anymore.
Manager IT Compliance, Fujirebio Diagnostics, Inc
Book a demoRevolutionize your validation
Digitalize validation your way, with the validation platform trusted by the world’s leading life sciences companies.
Talk to an expertCustomer Success
Case studies
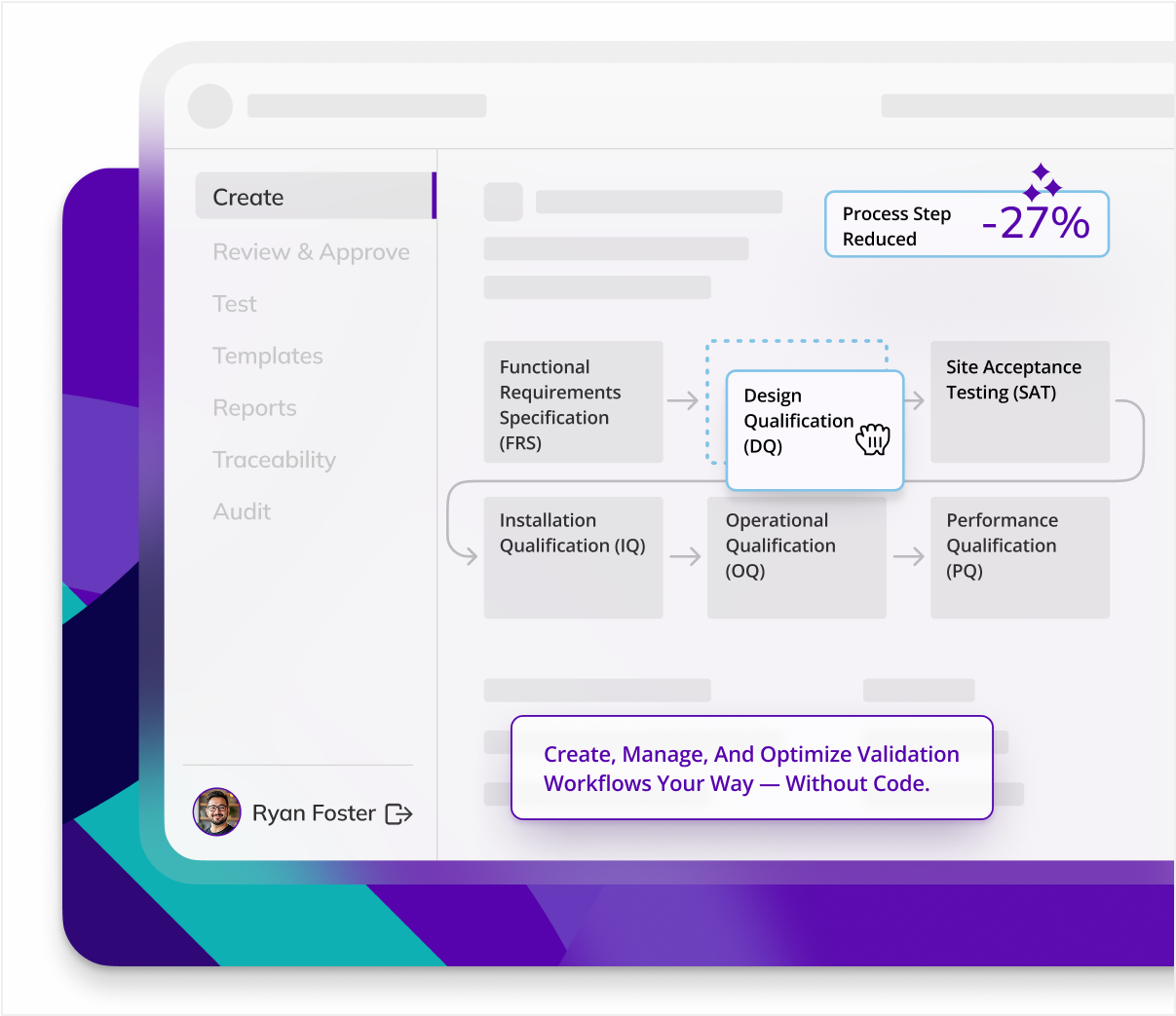
CSV Life - Cycle & Core Deliverables
CSV work begins long before testing and includes a large host of documents that detail user requirements, functionality, process plans, risk assessments, and more. Kneat Gx provides templates for every document you need and automatically builds a traceability matrix so you can link requirements, risks, and tests to get the document you need, when you need it.

Risk-Based CSV & Computer Software Assurance
The FDA advises a modern take on CSV, called Computer Software Assurance (CSA). This is a risk-based approach to validation which aligns with new GAMP 5 best practice. Under CSA, a company conducts validation efforts in proportion to the risk level of the system. Using Kneat and risk-based validation, Fujirebio Diagnostics Inc was able to cut CSV testing time by 46%.
| # | Software Category | Risk Level |
|---|---|---|
| 1 | Infrastructure Software | Lower Risk |
| 2 | Firmware (now an obsolete category) | N/A |
| 3 | Standard, Non-Configured Software | Lower Risk (Vendor) |
| 4 | Configurable, Off-the-Shelf (COTS) Software | Moderate to High Risk |
| 5 | Custom-Developed Software | High |
Which Systems Need Validation?
Any system related to Good Manufacturing Practices requires validation. The GAMP 5 guidance established four categories of system:
Kneat has a variety of features to streamline CSV, including template libraries, online test execution, risk, requirement, and test traceability, and the ability to leverage vendor tests and evidence to support risk assessment.
Results
CYCLE TIME REDUCTION
Kneat is proven to reduce cycle time by an average of 50% or more in multiple customer authored case studies.
CSAT Score
97% of our customers rate our customer support as either ‘Very Good’ or ‘Excellent.’
PLAN TO RENEW
Customer feedback via industry analyst SoftwareReviews.
Does Kneat support risk-based validation and the FDA’s Computer Software Assurance (CSA) model?
Yes. CSA is a named application area in Kneat Gx (“Computer System Validation (CSV) / Computer Software Assurance (CSA)”).
Are IQ/OQ/PQ templates built in?
Kneat lets you store pre-approved templates—including IQ, OQ and PQ—so teams can generate complete validation “document packs” quickly.
Can Kneat validate SaaS or other cloud-native systems?
Kneat is built to handle any validation requirement at any stage in the lifecycle. As such, it can validate other SaaS and cloud-native systems effectively.
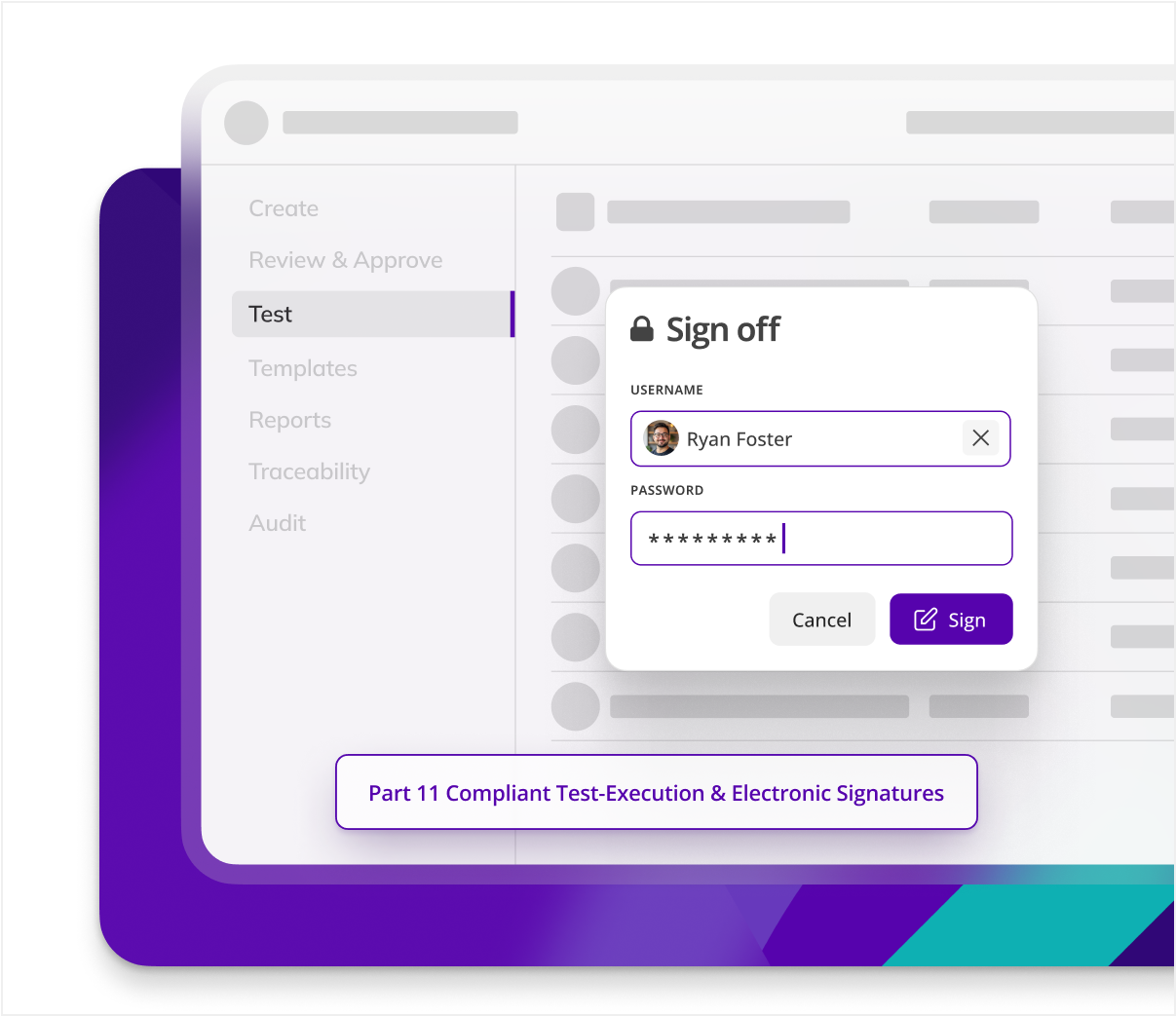
Does Kneat meet 21 CFR Part 11 & EU Annex 11 (e-signatures, secure audit trail)?
he platform is explicitly validated to Part 11/Annex 11, providing unique user IDs, electronic signatures, full version control, just to name a few features. Kneat Gx addresses every ALCOA++ principle and is built to secure compliance across the validation lifecycle.
Is there an open API to plug into ALM/QMS tools (e.g., Jira, SAP)?
Yes. Kneat exposes REST APIs for importing and exporting documents, drawings and data to external systems.
Can distributed teams review/approve remotely?
Yes—secure cloud access enables real-time, parallel or sequential remote reviews and approvals.