Cleaning validation plays a critical role in ensuring that equipment and facilities used in the production of pharmaceuticals and medical devices are free from contaminants. As the regulatory environment tightens and the complexity of products increases, organizations must stay on top of the latest trends to remain compliant and efficient. Here are seven trends shaping cleaning validation in 2024.
1. Digital Transformation and Automation
One of the most significant shifts in cleaning validation is the adoption of digital solutions. Digital transformation automates data collection, reducing manual errors, and increasing overall efficiency. In 2024, companies are increasingly leveraging digital validation systems to streamline their processes and enhance audit readiness.
According to the 2024 State of Validation: Essential Insights on Cleaning Validation whitepaper, 30% of survey respondents managing cleaning validation processes indicated they are currently using a digital validation system. Eighteen percent of respondents plan to adopt a digital validation system within the next year. Over one-third (38%) have plans to adopt digital validation systems within the next 1-2+ years. This reflects a sizeable shift towards digitalization in validation processes in 2024 and beyond (see chart below).
Digital Validation Adoption
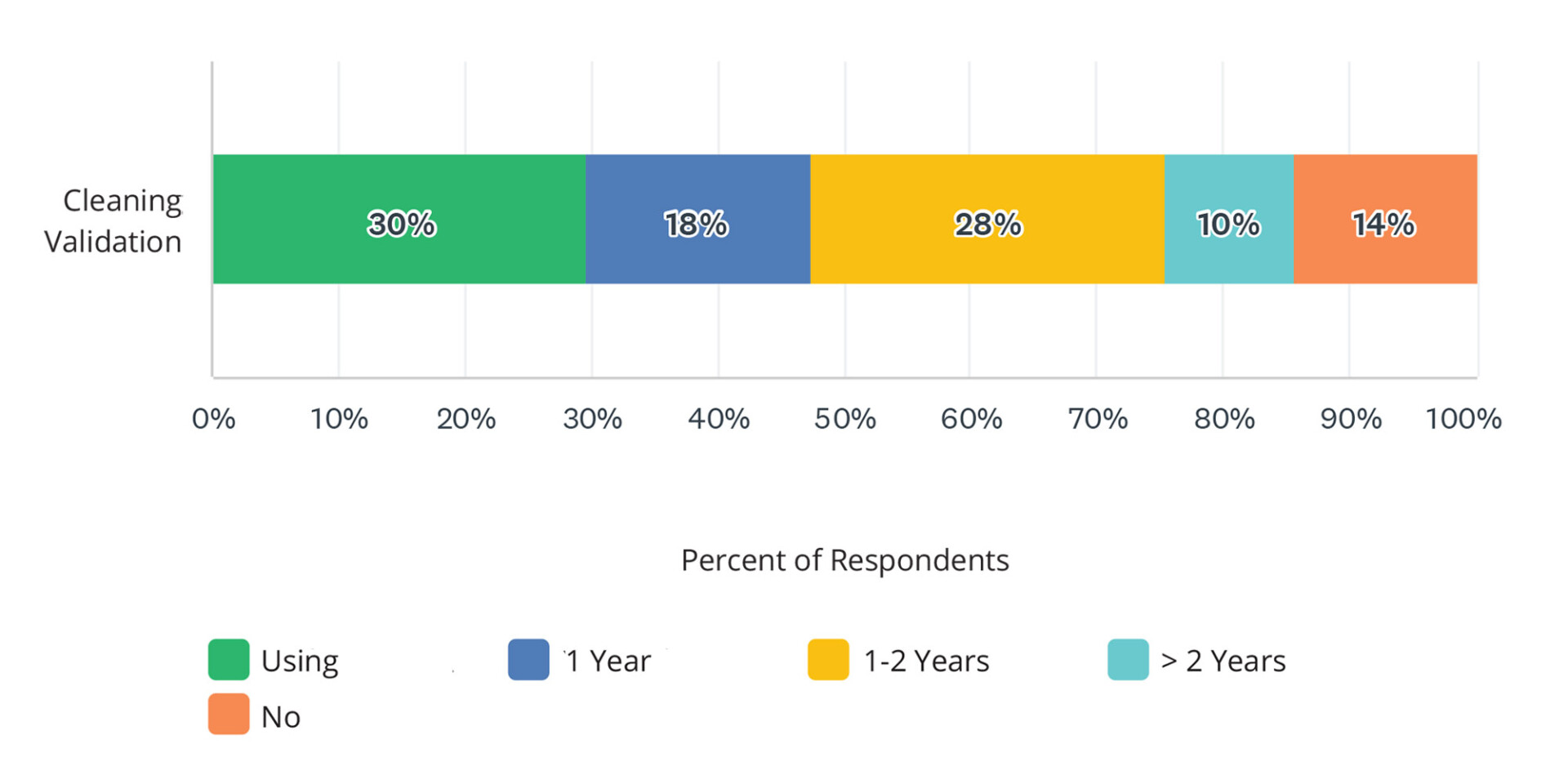
These systems enhance data integrity through features like automatic audit trails and electronic signatures, ensuring compliance with stringent regulatory requirements such as FDA’s 21 CFR Part 11 and EMA guidelines.
A key benefit of automation in cleaning validation is the reduction of cycle times. Digital systems can cut validation cycle times by up to 50%, making it easier for organizations to manage complex workflows and approvals remotely, all while maintaining a high level of accuracy and compliance.
2. Risk-Based Approaches
The shift towards a risk-based approach is another critical trend. Cleaning validation is increasingly driven by scientific risk assessments, particularly in shared manufacturing facilities where the risk of cross-contamination is high. Industry guidelines, such as ICH Q9 on Quality Risk Management, encourage manufacturers to adopt this approach to focus resources on the most critical cleaning processes.
Risk-based cleaning validation involves evaluating the potential for residual contamination based on the product’s properties, cleaning agents, and equipment used. This approach allows companies to reduce unnecessary testing and revalidation efforts, which translates into significant cost savings and resource efficiency.
3. Emphasis on Data Integrity
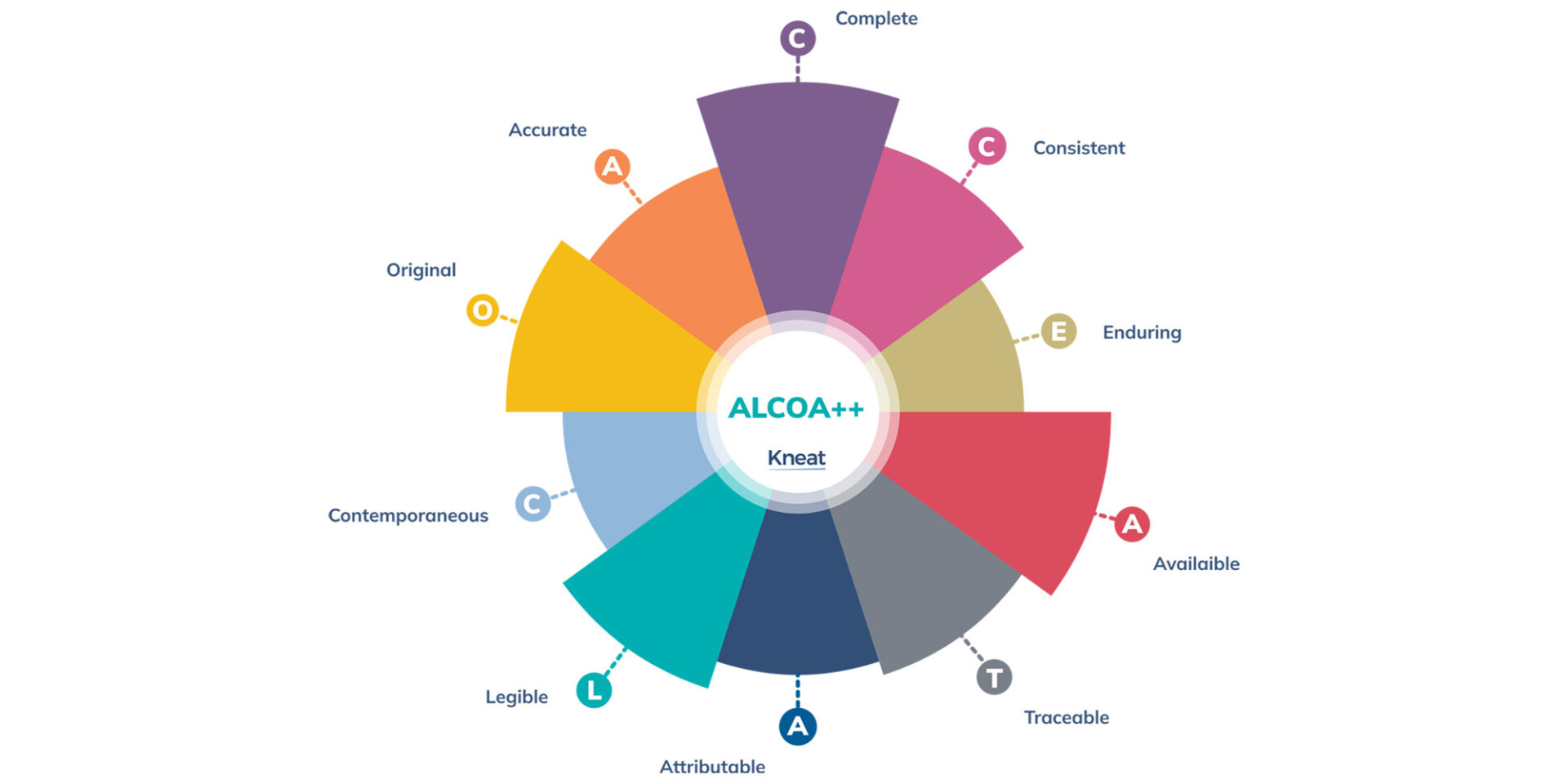
As regulations become more stringent, data integrity continues to be a focal point in cleaning validation. Ensuring that all data generated during validation is accurate, complete, and protected from unauthorized changes is critical for compliance.
Even those confident in their data integrity knowledge might be intrigued by a new concept that emerged in 2023: ALCOA++ . This enhanced framework, marked by the two plus signs, extends the core principles of data integrity.
The additional “+” represents a further emphasis on traceability. At a high level, a traceable document is one with a serviceable audit trail, with a clear line of activity which may include such actions as:
- When was the document reviewed last?
- How is it stored?
- Was the document revised?
- Why was the document revised?
As regulatory expectations rise with the advent of ALCOA++, ensuring the accuracy and traceability of your records is more essential than ever.
On-Demand Webinar: ALCOA++ and the Future of Data Integrity
In ALCOA++ and the Future of Data Integrity , data integrity expert Olivia Calder breaks down the ALCOA++ framework and what it means for the future of data integrity in validation processes. Learn how your organization can implement these principles effectively to ensure regulatory compliance and robust data governance.
With the increasing use of digital validation tools, companies are better positioned to maintain high data integrity standards through features like password-protected signatures and automated timestamps.
In addition to regulatory compliance, robust data integrity also supports better decision-making. By having access to reliable and readily available data, validation teams can quickly identify trends, optimize processes, and ensure continuous improvement.
4. Increased Outsourcing
Outsourcing is becoming a strategic component of cleaning validation, particularly for small and medium-sized organizations. According to the 2024 State of Validation: Essential Insights on Cleaning Validation whitepaper, 78% of organizations outsource some of their validation activities, with 32% outsourcing over a quarter of their validation efforts. The key drivers of this trend include the need for specialized expertise, cost savings, and flexibility.
Outsourcing allows companies to manage their workloads more effectively, especially when dealing with resource constraints or spikes in demand. It also offers access to cutting-edge technologies and expert knowledge, which can improve the efficiency and compliance of cleaning validation processes.
Download the 2024 State of Validation: Essential Insights on Cleaning Validation and discover the latest cleaning validation insights and trends from the 2024 State of Validation report, a comprehensive analysis of the global validation industry.
5. Continuous Monitoring and Real-Time Data Analysis
Continuous monitoring is increasingly becoming the standard for cleaning validation, especially in highly regulated environments. Advances in real-time data collection and analysis allow companies to continuously monitor critical cleaning parameters and adjust as needed. This reduces the need for frequent revalidation and ensures that equipment remains compliant over time.
Real-time monitoring also enhances process control, enabling companies to detect issues early and take corrective actions before non-compliance occurs. As technology advances, more organizations are expected to integrate continuous monitoring tools into their cleaning validation strategies. Both continuous monitoring and real-time data analysis become enabled through an organization’s digital transformation journey as systems and processes integrate and interconnect to utilize these tools for optimal efficiency.
6. Integration of Cleaning Validation into Broader Validation Programs
There is a growing trend of integrating cleaning validation with other validation processes, such as equipment and process validation. This holistic approach helps companies achieve greater operational efficiency by streamlining validation efforts across multiple departments. Integrated validation programs enable the use of shared data, reducing duplication of efforts and fostering a more collaborative environment.
Organizations are increasingly adopting software platforms, like Kneat Gx, that manage various types of validation under one system. This integration not only reduces complexity but also supports a more agile response to regulatory changes and audit requirements.
7. Sustainability and Resource Efficiency
Sustainability is gaining importance in the life sciences industry, and cleaning validation is no exception. Organizations are looking for ways to reduce their environmental impact by minimizing the use of paper, water, energy, and cleaning agents.
Newer technologies, such as automated cleaning systems and optimized cleaning cycles, are helping companies achieve these sustainability goals without compromising on compliance or safety.
This trend aligns with broader industry goals to reduce costs and improve the environmental footprint of manufacturing processes.
Final Thoughts
The cleaning validation landscape is evolving rapidly, driven by digital transformation, risk-based approaches, and an increasing focus on data integrity and sustainability. By staying informed about these trends, organizations can enhance their cleaning validation practices, ensure regulatory compliance, and improve overall operational efficiency.
Take your cleaning validation process to the next level with expert insights and practical strategies. Download the Digital Cleaning Validation Handbook today to discover how automation and data-driven approaches can enhance efficiency, compliance, and sustainability in your validation efforts.
Equip your team with the latest industry knowledge and optimize your cleaning protocols.





