The role of AI in pharma is revolutionizing drug discovery, drug development, and drug manufacturing. As pharmaceutical companies deploy advanced AI algorithms and AI technologies to accelerate new drug creation, the focus must shift to safety and validation. This guide explores key applications of AI and the critical need for artificial intelligence in pharmaceutical industry compliance. Discover how the use of AI is reshaping the landscape and how AI can help you balance rapid innovation with strict AI in pharma compliance.
For a deeper dive into these insights, watch our on-demand webinar, “Leveraging AI in Pharma: Navigating the Regulatory Landscape and Use Cases.”
Overview: how AI is transforming the pharma industry
Artificial intelligence in the pharmaceutical industry represents a fundamental shift in how pharma companies approach research and development, manufacturing, and patient care. The impact of AI extends across the entire pharmaceutical value chain, from early-stage drug discovery to post-market pharmacovigilance. AI is transforming traditional processes that once took years into streamlined workflows that deliver results in months.
The integration of AI technologies into pharma industries creates unprecedented opportunities for innovation. Pharmaceutical companies are increasingly recognizing that AI accelerates not only drug discovery but also clinical trials, manufacturing efficiency, and supply chain management. This wide-ranging application of artificial intelligence is revolutionizing the pharmaceutical industry in ways that were unimaginable a decade ago.
Defining artificial intelligence in pharma: from machine learning to generative AI
Artificial intelligence in pharma encompasses multiple technologies, each serving distinct functions in pharmaceutical research and development. Machine learning and artificial intelligence enable systems to analyze vast datasets, detect patterns, and make predictions without explicit programming. AI and ML algorithms can identify potential drug candidates by processing millions of molecular structures and predicting their physicochemical parameters using AI.
Generative AI represents a newer frontier in pharmaceutical product development. These AI systems can design novel molecular structures, optimize drug formulations, and even generate synthetic patient data for clinical trial planning. Natural language processing (NLP), another critical AI tool, extracts insights from unstructured medical literature and clinical notes, accelerating pharmaceutical research.
The application of artificial intelligence extends to artificial neural networks, which mimic human brain function to solve complex problems in drug development. These AI models learn from training data to recognize patterns in biological systems, predict drug efficacy, and identify potential adverse effects before human trials begin.
Industry 4.0 meets pharma 5.0: a new era for pharma industries
While Industry 4.0 introduced digitalization and automation into manufacturing, Pharma 5.0 emphasizes a human-centric, sustainable approach. AI sits at the heart of this evolution, enabling data-driven insights while addressing societal needs such as patient safety, equitable access, and environmental sustainability.
The integration of AI into drug development and pharmaceutical manufacturing marks the transition from traditional drug development methods to intelligent, adaptive systems. AI in the pharmaceutical industry enables real-time decision-making, predictive analytics, and personalized medicine approaches that define Pharma 5.0.
The role of artificial neural networks and AI tools in modern medicine
Artificial neural networks and advanced AI tools are reshaping modern medicine by enabling precision therapeutics and personalized treatment plans. These AI applications analyze patient genetics, medical history, and real-time health data to predict treatment responses and optimize therapeutic interventions.
Popular AI model tools used in pharma include deep learning frameworks for image analysis in pathology, predictive models for patient stratification in clinical trials, and AI algorithms that optimize pharmaceutical production schedules. The trending role of artificial intelligence in healthcare extends beyond treatment to prevention, with AI helping identify disease risks before symptoms appear.
AI for drug discovery and drug development
The implementation of artificial intelligence in drug discovery and development represents one of the most significant advances in pharmaceutical innovation. AI accelerates drug discovery by reducing the time required to identify promising compounds from years to months, fundamentally changing the economics of pharmaceutical research.
AI for drug discovery: accelerating the search for new compounds
AI in drug discovery transforms how pharmaceutical companies identify new drug candidates. Traditional drug discovery processes involved screening thousands of compounds through trial and error. AI algorithms can identify promising molecules by analyzing biological data, chemical properties, and existing research simultaneously.
AI for drug discovery uses machine learning models trained on vast chemical libraries to predict which molecular structures will bind effectively to disease targets. The drug discovery process benefits from AI’s ability to evaluate millions of potential compounds virtually, identifying the most promising candidates before expensive laboratory work begins.
AI accelerates drug discovery by predicting absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties early in development. This predictive capability helps pharmaceutical companies avoid costly late-stage failures and focus resources on compounds with the highest success probability.
AI in drug development: reducing time and cost in R&D
The drug development process traditionally takes 10-15 years and costs billions of dollars. AI in drug development dramatically reduces both timelines and expenses by optimizing multiple stages simultaneously. AI can optimize clinical trial designs, predict patient responses, and identify optimal dosing regimens before human trials begin.
Accelerating drug development through AI involves using predictive models to identify which patient populations will respond best to specific treatments. This precision approach reduces trial sizes, shortens timelines, and increases success rates. AI technologies analyze real-world evidence and historical trial data to inform better development strategies.
The integration of AI technologies into pharmaceutical research enables continuous learning systems that improve with each new data point. As more pharmaceutical products move through development, AI models become more accurate in predicting success, creating a virtuous cycle of improvement.
Generative AI applications in molecule design
Generative AI represents a breakthrough in how pharmaceutical companies approach new drug creation. These AI systems don’t just analyze existing compounds — they generate entirely novel molecular structures optimized for specific therapeutic targets. Generative AI can design molecules with desired properties while avoiding known toxicity patterns.
The use of artificial intelligence in molecule design enables researchers to explore chemical spaces that would never be discovered through traditional methods. AI presents millions of potential structures, evaluates their likely effectiveness, and prioritizes those with optimal drug-like properties. This capability transforms the early stages of pharmaceutical product development.
Beyond the lab: AI use in clinical trials and manufacturing
The applications of AI extend far beyond research laboratories into clinical development and pharmaceutical manufacturing. These practical implementations demonstrate how AI can help pharmaceutical companies improve efficiency, ensure quality, and maintain compliance across operations.
AI in clinical trials: optimizing patient recruitment and monitoring
AI in clinical trials addresses some of the pharmaceutical industry’s most persistent challenges. Patient recruitment remains a major bottleneck, with many trials failing to enroll sufficient participants. AI algorithms analyze electronic health records to identify eligible patients, dramatically reducing recruitment timelines.
AI is helping trial designers optimize protocols by analyzing historical trial data to predict enrollment rates, dropout risks, and optimal endpoint selection. Wearable AI devices enable continuous patient monitoring, collecting real-time data that provides richer insights than traditional periodic assessments. This continuous monitoring improves data quality while reducing patient burden.
The use of AI in trial monitoring also enhances safety oversight. AI systems can detect adverse event patterns across multiple trial sites simultaneously, enabling faster responses to emerging safety signals. This proactive approach protects patients while maintaining trial integrity.
AI in pharmaceutical manufacturing: predictive maintenance and quality
AI in pharmaceutical manufacturing transforms production from reactive to predictive. Predictive maintenance powered by AI analyzes equipment sensor data to anticipate failures before they occur, minimizing costly downtime. This application of artificial intelligence reduces waste, improves efficiency, and ensures consistent pharmaceutical product quality.
Drug manufacturing benefits from AI’s ability to optimize complex production parameters in real-time. AI systems adjust temperature, pressure, mixing speeds, and other variables to maintain optimal conditions, ensuring batch-to-batch consistency. The integration of AI into pharmaceutical manufacturing processes enables adaptive production that responds to variations while maintaining quality standards.
AI is used extensively in quality control, where computer vision systems inspect pharmaceutical products at speeds impossible for human inspectors. These AI tools detect defects, verify correct packaging, and ensure labeling accuracy with greater precision than traditional methods.
Our eBook Understanding AI in Life Sciences Quality Assurance and Validation explores how AI can transform your quality assurance and validation work, enhancing compliance, efficiency, and innovation at your organization.
AI use in pharmacovigilance and safety reporting
Pharmacovigilance represents a critical application where AI can also deliver significant value. AI is increasingly deployed to monitor adverse events across multiple data sources simultaneously — from social media and patient forums to electronic health records and regulatory databases. AI and data analytics enable pharmaceutical companies to detect safety signals faster than manual review processes.
Natural language processing AI tools extract relevant safety information from unstructured clinical notes, patient narratives, and medical literature. This automation accelerates adverse event reporting while improving data quality. AI helps pharmacovigilance teams prioritize cases requiring immediate attention, ensuring patient safety remains paramount.
The implementation of artificial intelligence in safety monitoring provides pharmaceutical and healthcare organizations with comprehensive visibility into product safety profiles. AI enables continuous monitoring rather than periodic reviews, shifting pharmacovigilance from reactive to proactive.
Challenges and opportunities of AI in pharma
While artificial intelligence in pharmaceutical industry applications offers tremendous potential, significant challenges and opportunities of AI must be addressed. Understanding these complexities enables pharmaceutical companies to implement AI responsibly while maximizing benefits.
Navigating the regulatory landscape: the EU AI Act & U.S. Executive Order
AI’s integration into pharma operations brings unique challenges, particularly around AI in pharma compliance. Two significant regulatory frameworks shape artificial intelligence in pharmaceutical industry compliance today: the EU Artificial Intelligence Act and the U.S. Executive Order 14110.
The EU AI Act
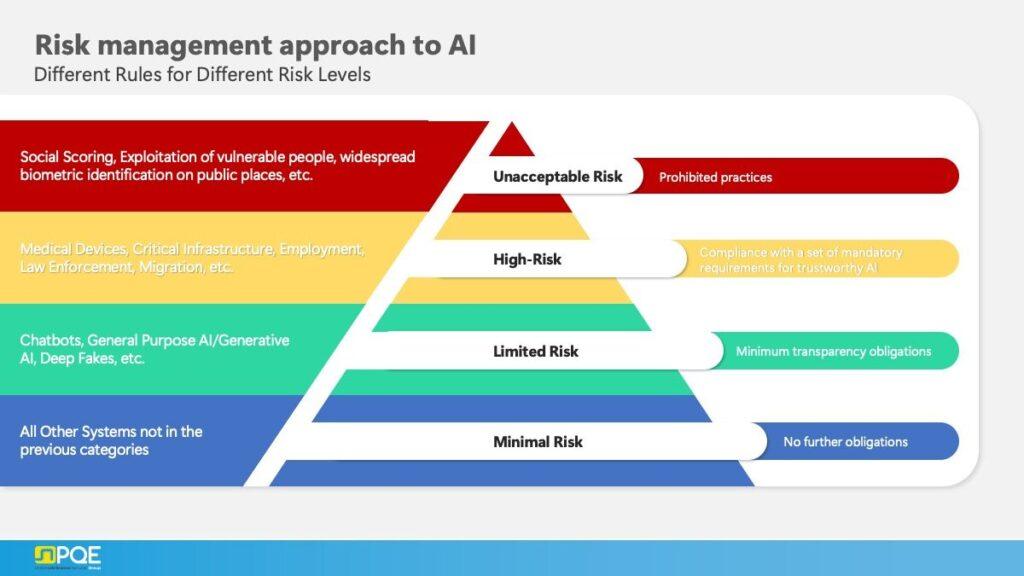
The EU AI Act categorizes AI systems into four risk levels: minimal, limited, high, and unacceptable. For high-risk systems, such as those impacting patient safety in pharmaceutical contexts, the Act mandates stringent requirements, including:
- Risk management systems
- Transparency and human oversight
- Continuous monitoring throughout the lifecycle
Non-compliance can result in penalties up to €40 million or 7% of global annual turnover. Many AI applications in pharmaceutical manufacturing and drug development fall into the high-risk category, requiring comprehensive validation and documentation.
U.S. Executive Order 14110
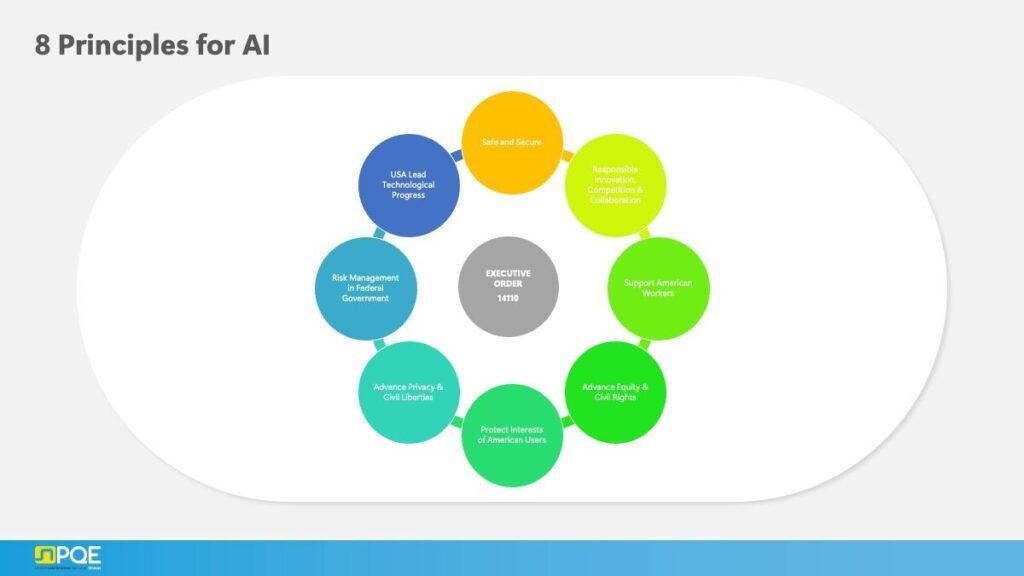
Signed in October 2023, this order emphasizes safety, transparency, and responsible AI development. It introduces eight guiding principles and requires large-scale AI developers to share critical safety information with federal agencies.
The principles emphasize:
- Safe and secure AI systems
- Responsible innovation, competition, and collaboration
- Support for American workers
- Advancement of equity and civil rights
- Protection of user interests and privacy
- Risk management in federal government applications
- U.S. leadership in technological progress
Both frameworks underscore the need for trustworthy AI systems with robust governance, data integrity, and human oversight. These regulations shape how pharmaceutical companies can integrate AI into their operations while maintaining compliance.
Data integrity and validation: the key to AI in the pharmaceutical industry
Data integrity represents the foundation of successful AI implementation in pharma. AI algorithms used to train AI models require high-quality, validated data to produce reliable results. Poor data quality leads to inaccurate predictions, potentially compromising patient safety and regulatory compliance.
The challenge of validating AI models stems from their complexity and adaptive nature. Unlike traditional software with predetermined outputs, AI models learn from data and may produce different results as they process new information. This dynamic behavior requires novel validation approaches that ensure AI systems remain within acceptable performance parameters.
Digital validation systems provide the framework needed to maintain AI in pharma compliance. These platforms track model performance, document training data lineage, and create audit trails that demonstrate ongoing compliance. Validation must address not just the AI algorithm itself but also the data pipelines, infrastructure, and human oversight processes that support it.
Overcoming barriers to AI adoption
Several barriers slow the implementation of artificial intelligence in pharmaceutical companies. Technical challenges include integrating AI with legacy systems and pharmaceutical supply chain management platforms. Cultural resistance often stems from concerns about job displacement and skepticism about AI’s reliability.
The potential of AI can only be realized when pharmaceutical companies address these barriers systematically. This requires investment in training, change management, and infrastructure modernization. Organizations must also develop governance frameworks that define appropriate AI use, establish accountability, and ensure ethical implementation.
AI could transform every aspect of pharmaceutical operations, but successful adoption requires more than technology — it demands organizational commitment to change. Companies that overcome these barriers position themselves to capture the full value of AI while maintaining regulatory compliance and operational excellence.
Key takeaways: the future of AI in pharma industry
The implications of AI for the pharmaceutical market extend beyond operational improvements to fundamental shifts in how medicines are discovered, developed, and delivered. As AI technologies mature, several key considerations emerge:
- Adopt a Risk-Based Approach: Understand the regulatory implications of AI systems and align them with risk categories defined by frameworks like the EU AI Act. High-risk applications in pharmaceutical production require more rigorous validation and oversight.
- Ensure Data Integrity: Implement robust data management practices to ensure AI-driven decisions are accurate and transparent. Data integrity forms the foundation of trustworthy AI in pharmaceutical operations.
- Foster Collaboration: Engage cross-disciplinary teams, including regulatory experts, data scientists, and process engineers, to navigate challenges and leverage AI effectively. The integration of AI requires expertise spanning multiple domains.
- Invest in Compliance: Develop Quality Management Systems (QMS) and post-market monitoring mechanisms to ensure ongoing compliance. AI in pharma compliance requires continuous attention, not one-time validation.
- Focus on Trustworthiness: Prioritize patient safety and ethical AI practices to build trust with stakeholders and regulators. The accuracy of AI models must be continuously verified against real-world outcomes.
AI is revolutionizing tasks in the pharmaceutical industry from discovery through manufacturing. AI plays an increasingly central role in pharmaceutical research, clinical development, and commercial operations. AI enables pharmaceutical companies to work faster, smarter, and more efficiently while maintaining the rigorous standards required in healthcare.
The various AI technologies available today — from machine learning to generative AI —offer different capabilities suited to specific challenges. AI has the potential to address longstanding pharmaceutical industry challenges while creating entirely new possibilities for therapeutic innovation. AI presents both tremendous opportunities and significant responsibilities for pharmaceutical companies.
Looking forward, the processes in pharmaceutical development will increasingly rely on AI-driven insights. Companies that embrace AI strategically, invest in proper validation, and maintain focus on patient safety will lead the next generation of pharmaceutical innovation. The help of AI in addressing complex biological questions and optimizing therapeutic interventions positions the pharmaceutical industry for unprecedented advances in human health.
Frequently asked questions
1. If an AI model ‘learns’ and changes over time, how can we possibly keep it validated without constant re-work?
This is the biggest challenge with “continuous learning” models. The regulatory solution is moving toward a Predetermined Change Control Plan (PCCP), where you pre-specify the range of allowable changes and establish boundaries for acceptable model behavior.
However, managing these dynamic changes on paper is impractical. Success requires a digital validation system that can automatically track incremental changes, monitor model performance metrics, and trigger risk assessments only when parameters drift outside your pre-set “safe zone.” This approach saves organizations from constant full re-validation while maintaining compliance.
Modern validation platforms log every model update, track performance against predefined acceptance criteria, and create audit trails that demonstrate continuous control. This ensures AI systems remain validated even as they adapt and improve over time.
2. Does using AI in manufacturing mean we have to replace our legacy equipment to stay compliant?
No. AI is often a layer on top of existing infrastructure rather than a replacement for it. For example, predictive maintenance sensors can be added to older machines without replacing the equipment itself. The compliance challenge typically isn’t the physical equipment — it’s ensuring the data integrity of the signals feeding your AI model.
You need a validation platform that can integrate with various data sources (MES, SCADA, or legacy PLCs) to create a “single source of truth.” This integration proves to auditors that the data feeding your AI model is accurate, original, and unmodified, meeting ALCOA++ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, Available, and Traceable).
Digital validation solutions bridge the gap between legacy systems and modern AI applications, enabling pharmaceutical companies to leverage AI without wholesale infrastructure replacement.
3. With the EU AI Act classifying many pharma tools as ‘High Risk,’ are we opening ourselves up to massive fines by adopting AI now?
The fines are real — up to €40 million or 7% of global annual turnover — but they punish lack of governance, not the use of AI itself. The “High Risk” classification simply means you need stricter documentation, human oversight, detailed logging, and transparency in how AI systems make decisions.
A digital validation workflow protects your organization by enforcing mandatory “human-in-the-loop” approval steps and automatically generating the granular audit trails that EU regulators explicitly demand. Rather than avoiding AI due to regulatory concerns, pharmaceutical companies should implement proper governance frameworks that enable compliant AI use.
The key is demonstrating that your AI systems are under control, validated for their intended use, and continuously monitored. Organizations with robust validation processes can adopt AI confidently while meeting regulatory requirements.
4. How do we explain ‘Black Box’ AI decisions to an auditor?
You don’t always need to explain the math inside the black box, but you must validate the outputs against known controls. This approach is called “Black Box Testing” or validating the intended use of the AI system.
The key is having a robust risk management framework linked directly to your testing data. If you can show an auditor a direct, traceable link between your Risk Assessment, your Test Protocols, and your AI system’s final outputs, you demonstrate control even if the algorithm itself is mathematically complex.
Documentation should focus on:
- The intended use and scope of the AI system
- Input data validation and quality controls
- Output verification against known standards
- Performance monitoring and acceptance criteria
- Human oversight and decision-making authority
This validation approach satisfies regulatory requirements without requiring deep mathematical explanations of neural network architectures or machine learning algorithms.
5. Won’t validating AI datasets take significantly longer than traditional equipment validation?
It can, if you treat data validation like equipment validation using paper-based processes. AI validation is data-centric, not document-centric, requiring a fundamentally different approach.
If you’re manually copy-pasting test results from Python or R into Word documents, validation will indeed take months. However, by switching to a paperless validation solution, you can automate the ingestion of test results, streamline review processes, and reduce cycle times by up to 50%.
Modern digital validation platforms integrate directly with data science tools, automatically capture test results, maintain data lineage, and generate validation reports. This automation ensures your validation speed keeps pace with your innovation speed, enabling rapid AI deployment without compromising compliance.
The key is selecting validation tools designed for the dynamic, data-intensive nature of AI systems rather than forcing AI validation into traditional equipment validation frameworks.
Transform your AI validation strategy
AI’s integration into the pharmaceutical sector is no longer a question of “if” but “how.” By embracing AI strategically and navigating the regulatory landscape effectively, pharmaceutical companies can unlock unprecedented value while maintaining the rigorous compliance standards required in healthcare.
At Kneat, we are committed to supporting this transition with Kneat Gx, our innovative digital validation solution. Our platform streamlines all validation processes, enhances data integrity, and supports innovation in the age of Pharma 5.0.
Book a personalized 1:1 demo today to see how our digital validation solution can help you implement AI confidently while maintaining regulatory compliance.







