Validation teams in the medical device sector are under more pressure than ever. Tighter regulations, complex product lifecycles, and the push toward digital transformation are reshaping the landscape. How are leading companies keeping pace? Our new whitepaper, Validation Strategies in Medical Devices , has the answers.
Validation challenges in the med device industry
Validation in the medical device sector is uniquely complex. Every product class — from low-risk Class I to high-risk Class III devices — brings a rising level of regulatory scrutiny, with strict adherence to standards such as FDA 21 CFR Part 11 and EU Annex 11.
Add to this the constantly evolving EU MDR requirements, multi-phase product lifecycles, and the need for continuous re-validation after even minor design or supplier changes, and it’s clear why validation remains a persistent challenge. Teams must not only maintain compliance but also balance speed-to-market, cost pressures, and innovation — all with lean resources and increasing workloads.
Want to dive deeper? Read our Medical Devices eBook Simplifying and Streamlining Medical Device Validation to explore the biggest validation challenges facing the industry — and how digital solutions are helping teams overcome them.
Insights from the latest industry data
The medical device industry is at a critical inflection point. According to the 2025 State of Validation study, which surveyed 300+ validation professionals worldwide, including a focused group of 96 respondents from the med device sector, teams are juggling more complexity, higher workloads, and growing compliance demands.
Here are some of the key insights you’ll uncover in the whitepaper:
1. Workloads are rising — but teams remain lean
Validation Team Size
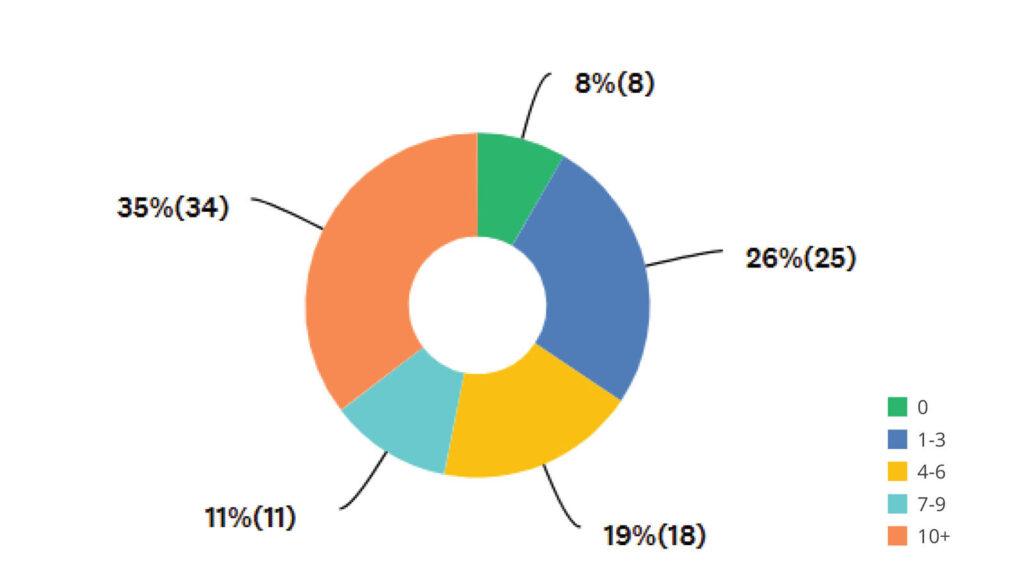
Nearly 70% of medical device professionals report increasing validation workloads in 2025, but more than half of teams still have six or fewer members. This mismatch highlights the urgent need for smarter processes and digital support.
2. Digital validation adoption is accelerating
Digital Validation Adoption Status
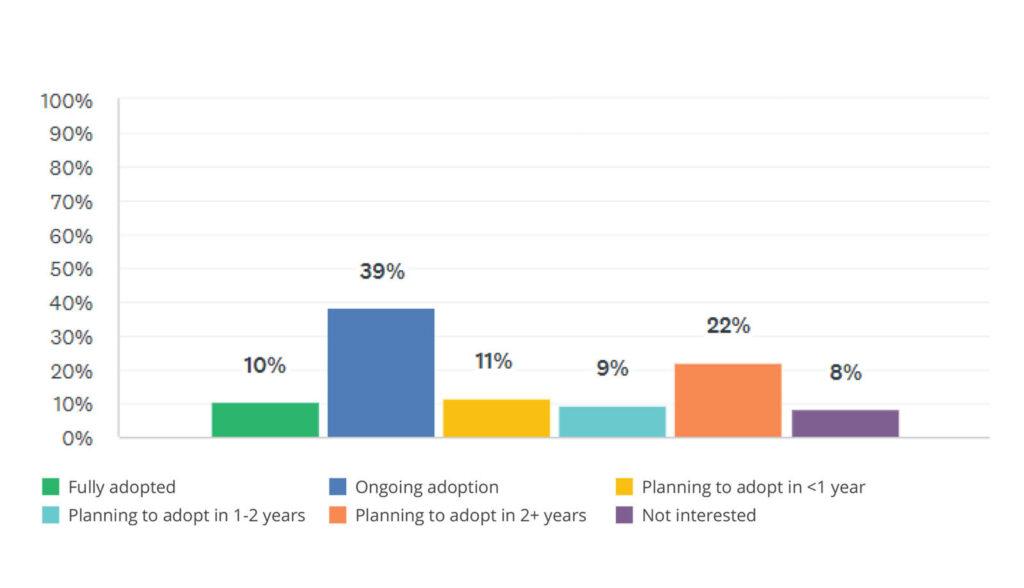
Half of med device organizations have fully adopted or are actively implementing digital validation systems and early adopters are seeing strong ROI. Benefits such as data integrity, audit readiness, and global standardization are driving digital’s rise as a strategic necessity.
3. CSA and AI: the next frontier
AI Adoption Status
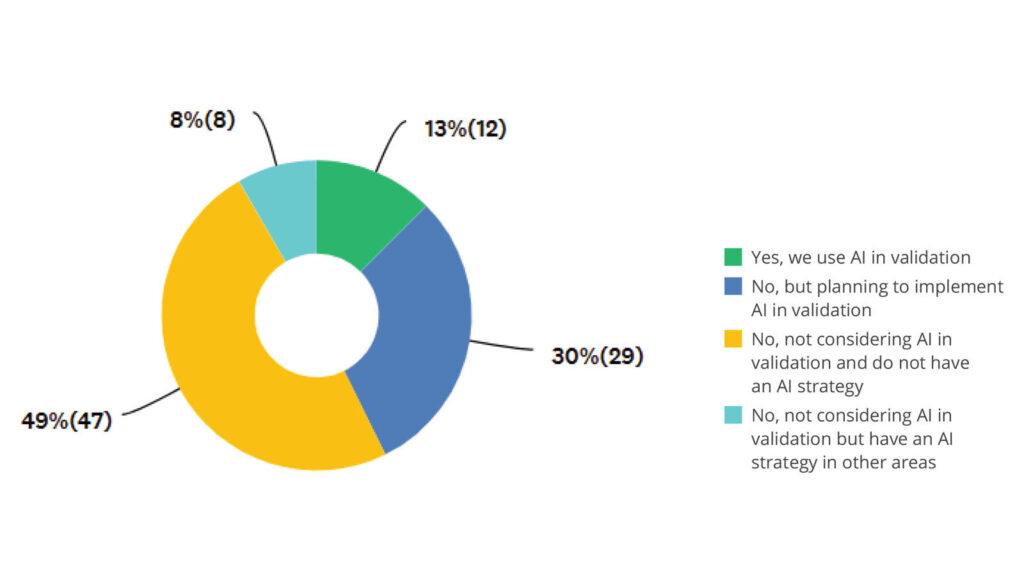
Computer Software Assurance (CSA) is slowly gaining traction, but only 16% of organizations have fully adopted it. At the same time, AI remains in its exploratory phase — with just 13% currently using it — but confidence is high that it will become standard within five years.
4. Integration and change management are key
Integration with Other Systems
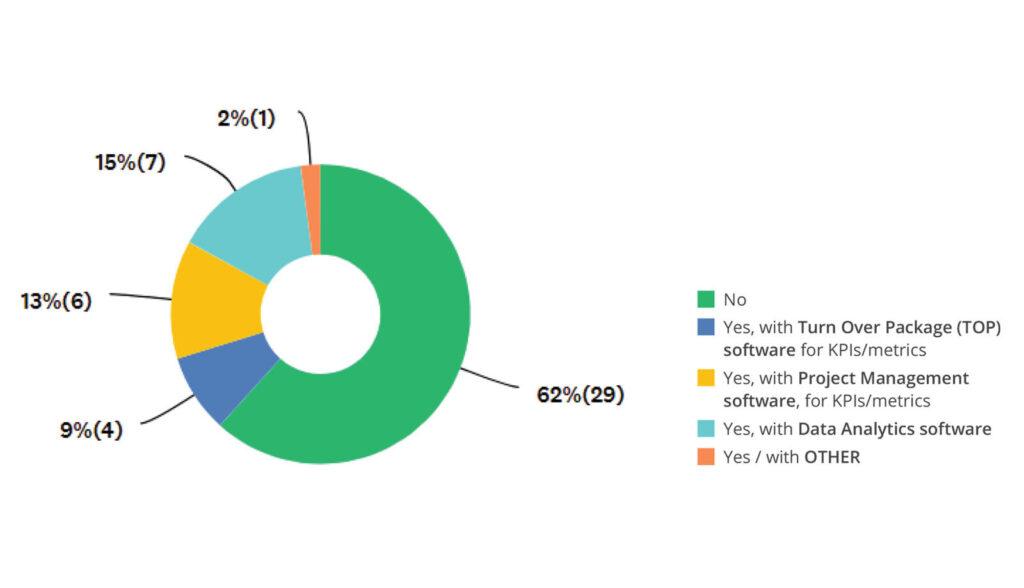
While digital transformation brings measurable returns, integration challenges and resistance to change are slowing progress. Successful organizations pair technology with robust change management strategies to drive sustainable adoption.
Why you should read the whitepaper
This whitepaper goes beyond high-level trends, offering practical recommendations for med device validation teams:
- How to build a digital-first strategy aligned with audit readiness and efficiency goals.
- Steps to strengthen CSA readiness through education and expert support.
- Guidance for piloting AI safely and effectively in low-risk workflows.
- Ways to unlock greater value through system integration.
Get your copy
Equip your validation team with the latest data, expert analysis, and actionable strategies for an evolving medical device landscape.







