Top 10 Validation Challenges for Life Sciences SMBs in 2022
We surveyed medium sized pharmaceutical and biotechnology companies in the United States and Europe to uncover their top ten validation challenges, here’s what we found.
Contrary to its ‘big-pharma’ reputation, the life sciences industry is comprised mostly of smaller companies, with more than 90% of the industry constituted by small to medium size businesses (SMBs) generating less than $1 billion in sales. Life sciences SMBs are growing quickly too, with revenue growth reaching a strong 6.9% in 2020.
Despite this, most industry thought leadership, insight and solutions focus on the challenges and opportunities of larger organizations. To better understand the validation challenges of medium sized companies, we surveyed a group of pharmaceutical and biotechnology organizations in the United States and Europe to uncover their top ten validation challenges – here’s what we found.
Our survey results
The top-10 validation challenges facing SMBs today
Ranked from most to least important, the top ten validation challenges facing medium sized companies today are: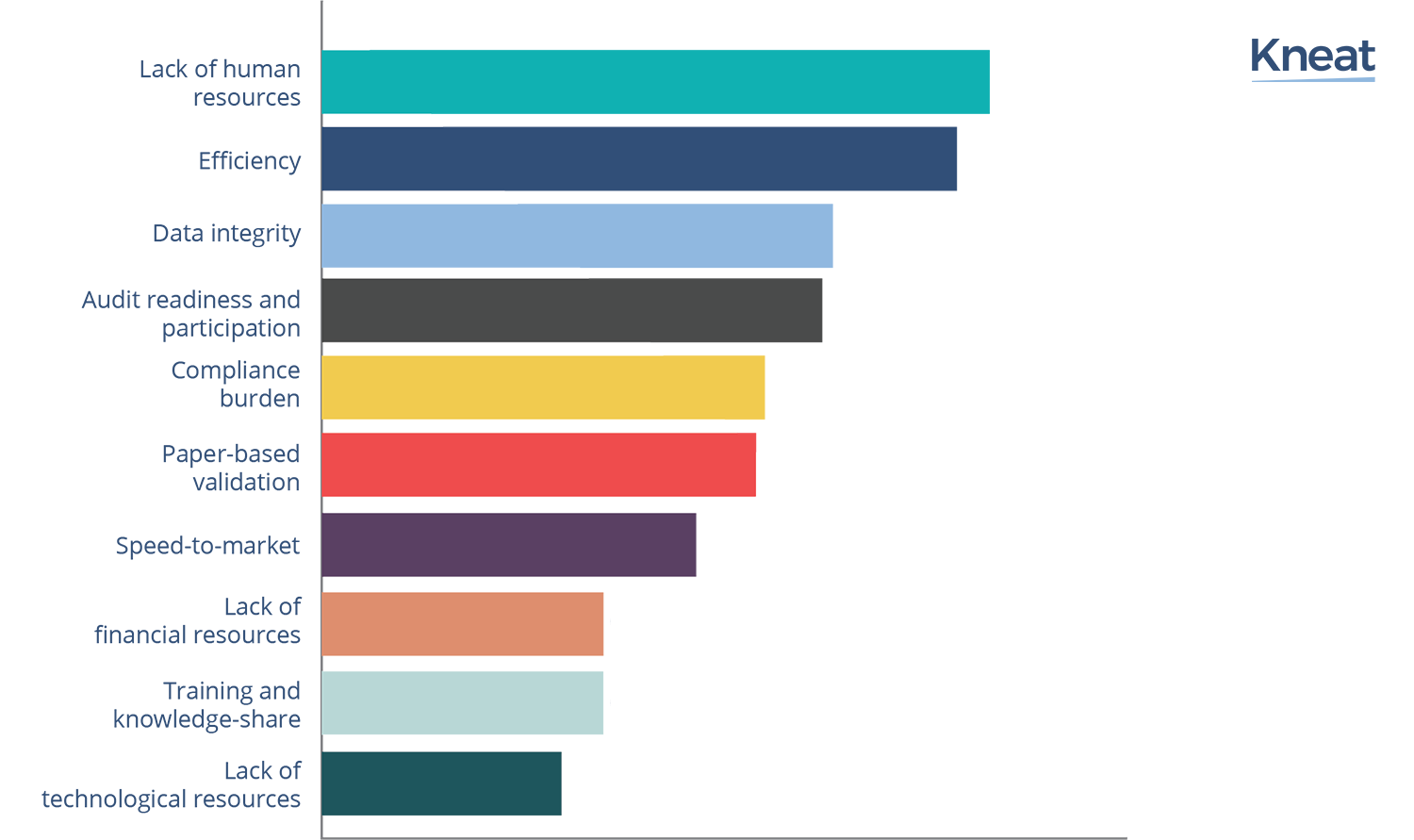
‘Lack of human resources was ranked overall in pole position, having been ranked in the top three by 86% of respondents. Validation in the Life Science industry is a highly knowledge-driven and technology-based process that relies heavily on its human resources. So perhaps it is not surprising that smaller organizations’ validation and quality assurance experts find ‘having to do more with less’ particularly onerous.
Read the full eBook

For our full survey results and more insight into the challenges facing life sciences SMBs and insight into how to overcome them, read our e-Book Overcoming Validation Challenges for SMB’s.
• Understand the validation challenges unique to life sciences SMBs
• Gain insight into the impact regulatory burden is having on the sector
• Learn ways to address these challenges
About our survey
Our respondents replied to an online survey conducted between January 18 and January 25, 2022. Respondents were comprised of manager level up professionals working in validation and quality assurance functions, of small to medium sized pharmaceutical and biotechnology companies located in the United States and Europe.
Introducing Kneat Go – digitize faster, on-budget
Experience the full power of Kneat, in a services bundle tailored to the requirements and budget of highly regulated, medium-sized companies, with Kneat Go. Kneat Go’s fully qualified installation and streamlined project documentation allows you to go-live in just weeks, delivering an even faster return on investment.
Digitize validation faster, on-budget, with Kneat Go.
What’s included?
✓ The latest, most powerful version of Kneat, forever
Enjoy the full power of Kneat, including all new releases, in a bundle tailored to the needs and budgets of medium-sized companies.
✓ 10 concurrent licenses, covering up to 50 registered users
The ability to share one license between up to five users.
✓ Power User Level 1 Kneat Academy Training for up to eight ‘Admin’ level users
World-class training for team leaders and other senior Kneat users.
✓ Single tenant low-latency SaaS infrastructure (private, dedicated database)
Your own, highly secure, private high-speed database.
✓ Two system environments including Development and Production
Make changes to your system without affecting active users.
✓ Fully pre-qualified installation
Kneat is a pre-qualified system, enabling faster validated installation.
✓ Streamlined project documentation
Off-the-shelf project documentation, with no approval-cycle
✓ Free automated patch updates
Continuous improvement of your latest Kneat Gx version.
✓ Graduated support model
Rapid-response external support for escalated tickets.
✓ Simplified default system configuration, with optional add-ons
Digitize faster, with the option to implement features like single-sign-on (SSO)
Why companies of all sizes choose Kneat
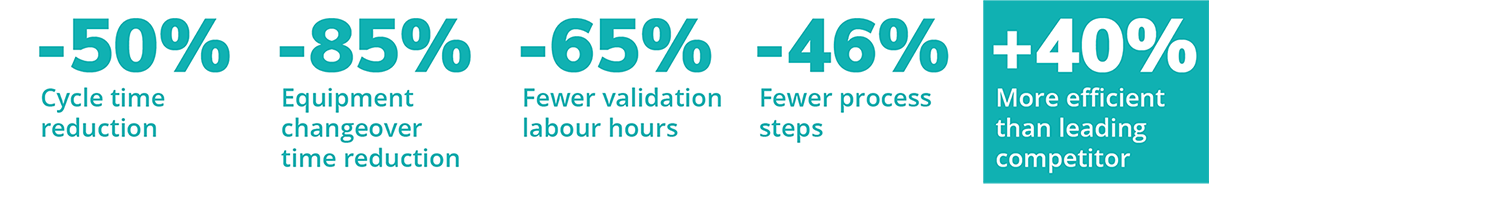
Digitizing the validation life cycle revolutionizes the speed, accuracy, intelligence, transparency, and management of validation processes. No matter your company’s resources or scale, you can achieve quality assurance best practices, improve productivity, and reduce your time-to-market, changeover, and cycle-times with Kneat.
 We worked closely with the life sciences industry to develop Kneat Go to help medium-sized companies harness the full power of Kneat, faster, and on-budget. Go live sooner, scale seamlessly as you grow, and receive world-class training to develop your own in-house Kneat subject matter experts for rapid support, administration, and code-free new process setup.
We worked closely with the life sciences industry to develop Kneat Go to help medium-sized companies harness the full power of Kneat, faster, and on-budget. Go live sooner, scale seamlessly as you grow, and receive world-class training to develop your own in-house Kneat subject matter experts for rapid support, administration, and code-free new process setup.
“Implementing Kneat…has enabled us to streamline our work processes and provide a more efficient and effective way to meet the increasing demands of the business for compliance and productivity.
– Quality Director, Project Sponsor and Champion, Myriad Genetics
Learn more in our webinar
Register today for our webinar “Overcoming Validation Challenges for SMBs” to discover more about the validation challenges of small to medium sized companies and how to resolve them faster, for less on-budget – with Kneat Go.
Our live webinar takes place on Wednesday, February 23, 2022 at 15:30-16:30 GMT / 10:30-11.30 ET.
Contact
Talk to us
Find out how Kneat can make your validation easier, faster, and smarter.
Start your paperless validation revolution by speaking to our experts.

