Access GxP and non-GxP documents in one secure, compliant platform to reduce barriers for product teams so they can focus on enhancing product quality and accelerating production.
Why Digitalize Document Management
Kneat Gx has always provided intelligent documents for online test execution, central data management, and smart traceability within a 21 CFR Part 11/Annex 11 compliant platform. These documents are Kneat native documents as they are created within Kneat Gx. Now you can also manage the lifecycle of non-Kneat native documents such as policies, procedures, reports, True Copy scans, spreadsheets, and engineering documents within the same platform, thanks to Kneat’s Document Management module.
As Kneat has already proven for Kneat native documents, digitalizing your documents results in significantly reduced cycle times, enhanced data integrity and compliance, deeper data insights, and lower costs to companies. Digitalizing your non-Kneat native documents is equally important. If a business needs to sign or find a business document, then it needs a digitalized document management system.
Kneat’s Document Management Module
The Kneat Document Management module delivers effective, efficient, and compliant management of non-native Kneat documents, both GxP and non-GxP, throughout their lifecycle to live alongside native Kneat documents in Kneat Gx.
Users create their documents in their document generation software apps (Word, PDF, Excel); check-in to Kneat Gx for review and redlining; check-out from Kneat Gx into the relevant software app for updates; check-in updated documents to Kneat Gx for review and compliant e-signature approval.
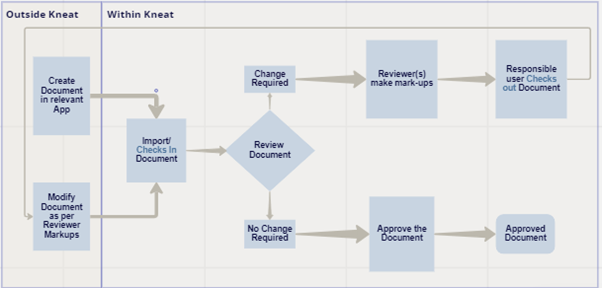
Key Features
Kneat’s Document Management module provides a range of benefits to companies, including:
- 21 CFR Part 11/Annex 11 compliant document management, complete with e-signature, access control, configurable workflows, version control, backup storage, and audit trail
- Controlled document check-out/check-in, with manual/bulk upload of various file types such as Word, Excel, PowerPoint, PDF, and JPEG
- Online collaborative comprehensive review of documents e., redlining with annotation
- Search for any document by number, title, project, system, etc
- Configurable access restrictions in line with business needs
- Cross reference and link non-native Kneat documents within Kneat-native documents
- Integration with other systems
- Migrate existing documents from an external system into Kneat DMS
- Synchronize, uni or bi-directional integration with another system
Kneat Gx: End-to-End Digital Validation
Document management is one function of Kneat’s complete digital validation offering, Kneat Gx. The Kneat platform also enables full end-to-end digitalization of all your validation processes, provides validation and quality teams with the ability to save time and money by digitalizing all validation processes to enhance business intelligence, increase compliance, and mitigate risk.
Request Your Demo Today!
Other Applications
Use Kneat to manage any validation, commissioning or qualification process.
- Kneat Gx | Audit Readiness
- Drawing Management
- Electronic Logbook Management
- Analytical Instrument Validation
- Utility and Facility Validation
- Process Validation
- Method Validation
- Equipment Validation
- Computer System Validation (CSV)
- Commissioning and Qualification
- Cold Chain Validation
- Cleaning Validation
Contact
Talk to us
Find out how Kneat can make your validation easier, faster, and smarter.
Start your paperless validation revolution by speaking to our experts.
