Use case
Commissioning and qualification
Ensure facilities, systems, and equipment are designed, installed, tested, and operated per requirements — as efficiently as possible.





The Trusted Digital Validation Platform
Proven to deliver data integrity, efficiency, and value on a global scale.
TRUSTED BY
Customer Ratings
G2
Overall rating by customers via leading software review site, G2.
CSAT Score
97% of our Customers rate our Customer Support as ‘Very Good’ or ‘Excellent’.
PLAN TO RENEW
Customer feedback via industry analyst SoftwareReviews.com.
Customer Success
Why Kneat
Digitalize any validation process in one platform, your way. Assure integrity, optimize efficiency, and be audit ready.
Assure data integrity, traceability, and compliance across the entire validation lifecycle.

Digitalize any validation process your way in one easy-to-use, highly configurable no-code solution.
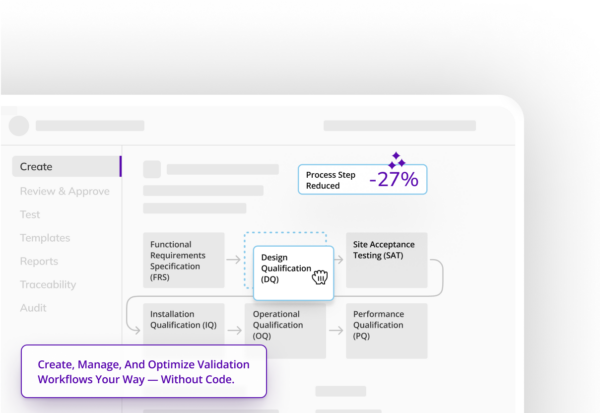
Be audit ready all the time. Manage audits in real time and share documents instantaneously.
Proven to reduce cycle times, protocol completion times, and process steps without compromising quality.
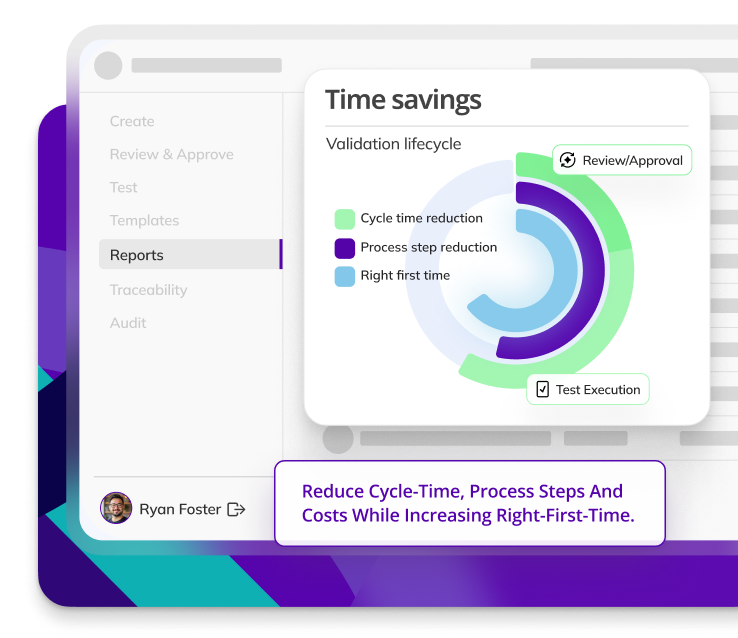
97% of Customers rate our Customer Support as 'Very Good' or 'Excellent.'
Use Cases
A single platform for commissioning and qualification, equipment validation, computer system validation, and more.
Use case
Ensure facilities, systems, and equipment are designed, installed, tested, and operated per requirements — as efficiently as possible.

Use case
Ensure computer systems are validated, tested, and maintained in compliance with requirements. Guaranteeing efficiency and effectiveness throughout their lifecycle.

Use case
Ensure equipment is validated, tested, documented, and maintained to meet operational and regulatory requirements. Assuring consistent and efficient performance.

Use case
Ensure analytical instruments are qualified, calibrated, and maintained to provide accurate, reliable, and traceable results.

Use case
Maintain a state of continuous compliance and documentation accuracy to ensure preparedness for regulatory inspections and all forms of audits.
